Tuesday Poster Session
Category: Liver
P6007 - An Unusual Case: Reishi Mushroom Supplement Unveiling Autoimmune Hepatitis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KH
Kathryn Hobbs, MD (she/her/hers)
Inova Fairfax Medical Campus
Fairfax, VA
Presenting Author(s)
Kathryn Hobbs, MD1, Dipam Shah, MD2, Showkat Bashir, MD2
1Inova Fairfax Medical Campus, Fairfax, VA; 2INOVA Fairfax Hospital, Fairfax, VA
Introduction: Reishi mushroom (Ganoderma lucidum) is an herbal supplement that is often touted for its potential immune-boosting and anti-inflammatory properties. However, the use of herbal supplements is not without risks and adverse effects can occur. In our literature search, there have been three cases, one fatal, of severe hepatocellular injury following reishi mushroom ingestion. We present a case of autoimmune hepatitis unmasked by reishi supplementation.
Case Description/
Methods: A 42-year-old female presented with abdominal pain, jaundice, dark urine, and pale stools approximately 1 month after initiating reishi mushroom supplements for acne. No other hepatotoxic prescriptions were identified. Initial labs showed elevation in LFTs (AST 1361, ALT 1644, ALP 186, and bilirubin 6.6). Viral hepatitis panels and drug screens were negative. Imaging revealed periportal and gallbladder wall edema, along with a likely hepatic hemangioma. Further labs tests showed positive ANA (1:320); although other antibodies (ASMA, LMK-1, AMA) were negative. Liver biopsy revealed severe acute hepatitis with parenchymal injury. Initial treatment with N-acetylcysteine (NAC) was ineffective. Given persistent dysfunction, Prednisone 40mg daily was initiated after biopsy, resulting in significant improvement in LFTs. Post-discharge, attempts to taper off steroids led to a rise in LFTs, requiring re-initiation of high dose steroids. Azathioprine was trialed but not tolerated. The patient remains on corticosteroids.
Discussion: Reishi mushroom’s hepatotoxic potential remains under-investigated. In this case, the patient developed acute liver injury following the ingestion of reishi mushroom supplements. The temporal relationship between supplement intake and the onset of symptoms suggests a causal link. The patient's recurrent rise in LFTs following steroid taper strongly suggests the presence of an underlying autoimmune hepatitis rather than isolated herb-induced hepatotoxicity. This case highlights the importance of prompt recognition and intervention in suspected herb-induced hepatotoxicity. Further research is needed to establish safety guidelines for reishi use.
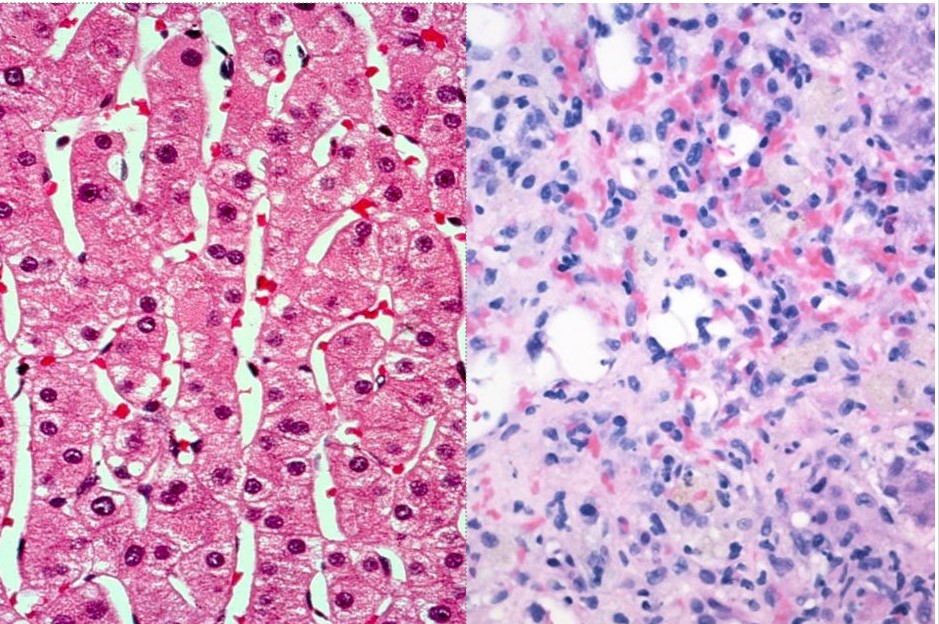
Figure: Normal (left) vs Confluent Necrosis (right)
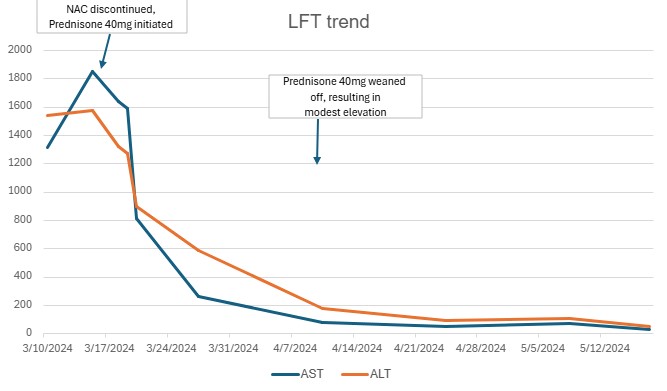
Figure: LFT trend
Disclosures:
Kathryn Hobbs indicated no relevant financial relationships.
Dipam Shah indicated no relevant financial relationships.
Showkat Bashir indicated no relevant financial relationships.
Kathryn Hobbs, MD1, Dipam Shah, MD2, Showkat Bashir, MD2. P6007 - An Unusual
Case: Reishi Mushroom Supplement Unveiling Autoimmune Hepatitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Inova Fairfax Medical Campus, Fairfax, VA; 2INOVA Fairfax Hospital, Fairfax, VA
Introduction: Reishi mushroom (Ganoderma lucidum) is an herbal supplement that is often touted for its potential immune-boosting and anti-inflammatory properties. However, the use of herbal supplements is not without risks and adverse effects can occur. In our literature search, there have been three cases, one fatal, of severe hepatocellular injury following reishi mushroom ingestion. We present a case of autoimmune hepatitis unmasked by reishi supplementation.
Case Description/
Methods: A 42-year-old female presented with abdominal pain, jaundice, dark urine, and pale stools approximately 1 month after initiating reishi mushroom supplements for acne. No other hepatotoxic prescriptions were identified. Initial labs showed elevation in LFTs (AST 1361, ALT 1644, ALP 186, and bilirubin 6.6). Viral hepatitis panels and drug screens were negative. Imaging revealed periportal and gallbladder wall edema, along with a likely hepatic hemangioma. Further labs tests showed positive ANA (1:320); although other antibodies (ASMA, LMK-1, AMA) were negative. Liver biopsy revealed severe acute hepatitis with parenchymal injury. Initial treatment with N-acetylcysteine (NAC) was ineffective. Given persistent dysfunction, Prednisone 40mg daily was initiated after biopsy, resulting in significant improvement in LFTs. Post-discharge, attempts to taper off steroids led to a rise in LFTs, requiring re-initiation of high dose steroids. Azathioprine was trialed but not tolerated. The patient remains on corticosteroids.
Discussion: Reishi mushroom’s hepatotoxic potential remains under-investigated. In this case, the patient developed acute liver injury following the ingestion of reishi mushroom supplements. The temporal relationship between supplement intake and the onset of symptoms suggests a causal link. The patient's recurrent rise in LFTs following steroid taper strongly suggests the presence of an underlying autoimmune hepatitis rather than isolated herb-induced hepatotoxicity. This case highlights the importance of prompt recognition and intervention in suspected herb-induced hepatotoxicity. Further research is needed to establish safety guidelines for reishi use.

Figure: Normal (left) vs Confluent Necrosis (right)

Figure: LFT trend
Disclosures:
Kathryn Hobbs indicated no relevant financial relationships.
Dipam Shah indicated no relevant financial relationships.
Showkat Bashir indicated no relevant financial relationships.
Kathryn Hobbs, MD1, Dipam Shah, MD2, Showkat Bashir, MD2. P6007 - An Unusual
Case: Reishi Mushroom Supplement Unveiling Autoimmune Hepatitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

