Monday Poster Session
Category: Interventional Endoscopy
P3541 - Real World Assessment of Post-ERCP Infections From a Quality Dashboard
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TA
Tareq Alsaleh, MD
Department of Internal Medicine, AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Tareq Alsaleh, MD1, Sanjeevani Tomar, MD2, Abdullah Abbasi, MD3, Saurabh Chandan, MD3, Sagar J.. Pathak, MD3, Maham Hayat, MD3, Deepanshu Jain, MD3, Kambiz Kadkhodayan, MD3, Dennis Yang, MD, FACG4, Mustafa Arain, MD3, Irteza Inayat, MD5, Muhammad Hasan, MD, FACG3, Natalie Cosgrove, MD3
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 3Center for Interventional Endoscopy, AdventHealth Orlando, Orlando, FL; 4Center for Interventional Endoscopic, AdventHealth Orlando, Orlando, FL; 5Department of Gastroenterology and Hepatology, Adventhealth Orlando, Orlando, FL
Introduction: Endoscopic Retrograde Cholangiopancreatography (ERCP) is associated with risks including infections, particularly in high-risk scenarios. American Society for Gastrointestinal Endoscopy (ASGE) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines for ERCP antibiotic prophylaxis (AP) differ and are somewhat vague. Our center monitors ERCP outcomes using a Quality Dashboard. A review of post ERCP infections was performed to assess accuracy, guideline adherence, and identify areas for improvement.
Methods: Two quality data abstractors reviewed electronic medical records (EMRs) of all ERCP at a quaternary referral center between April 1, 2024, to May 22, 2025 as part of post ERCP quality outcome monitoring, and conducted follow-up calls at days 3-7 and 14-30 post procedure. Cases of suspected infection within 14 days of ERCP were assessed for adherence to guidelines and data collection accuracy.
Results: Abstractors noted 40 cases of post-ERCP infection out of 3,035 total ERCPs (1.32%). After physician review, abstractors were accurate in 36 out of 40 assessments (90%). Abstractor false positives included 3 pre-ERCP infections and 1 case of post-ERCP abdominal pain with SIRS.
In the 36 cases of true post-ERCP infection, AP was administered on the same day as ERCP in 10 procedures (27.8%). Among these, 3 (30%) received only peri-ERCP antibiotics, while 9 (90%) received antibiotics both peri-ERCP and for several days after. For 2 cases of true post-ERCP infection, patients were on antibiotics that were not considered AP (Daptomycin, Rifampin).
Most ERCPs with AP were inpatient procedures (7 out of 10, 70%), while most ERCPs without AP were outpatient procedures (17 out of 26, 65.4%) (Table 1). There was a lack of AP guideline adherence to either ASGE or ESGE guidelines in 14 cases of post-ERCP infections (38.9%). Most of those cases occurred in female patients and in the outpatient setting (Table 2).
Discussion: Post ERCP infection reporting by data abstractors was reasonably accurate, although false positives were noted. While infection rates were overall low, several cases were identified which may have been mitigated by following available guidelines, presenting opportunity for quality improvement. Additionally, infections attributed to post ERCP occurred in appropriately treated patients, reflecting potential limitations of the current guidelines. Enhanced guideline adherence, improved abstractor training, and refined risk stratification are warranted.
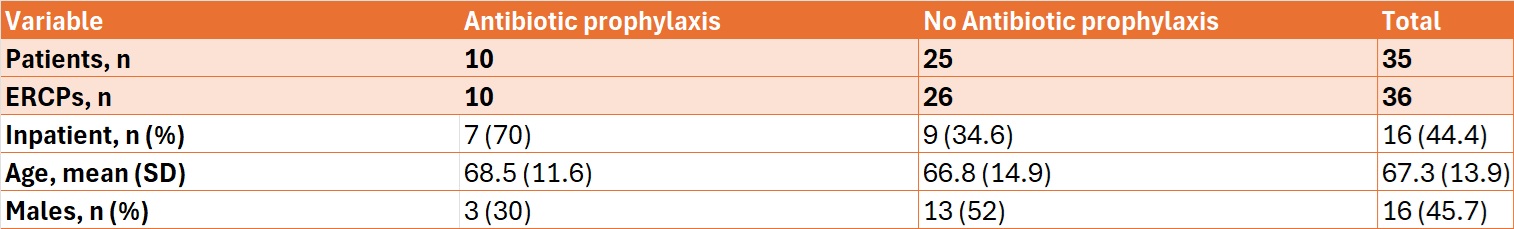
Figure: Table 1. Patient Characteristics for post-ERCP infections with and without antibiotic prophylaxis
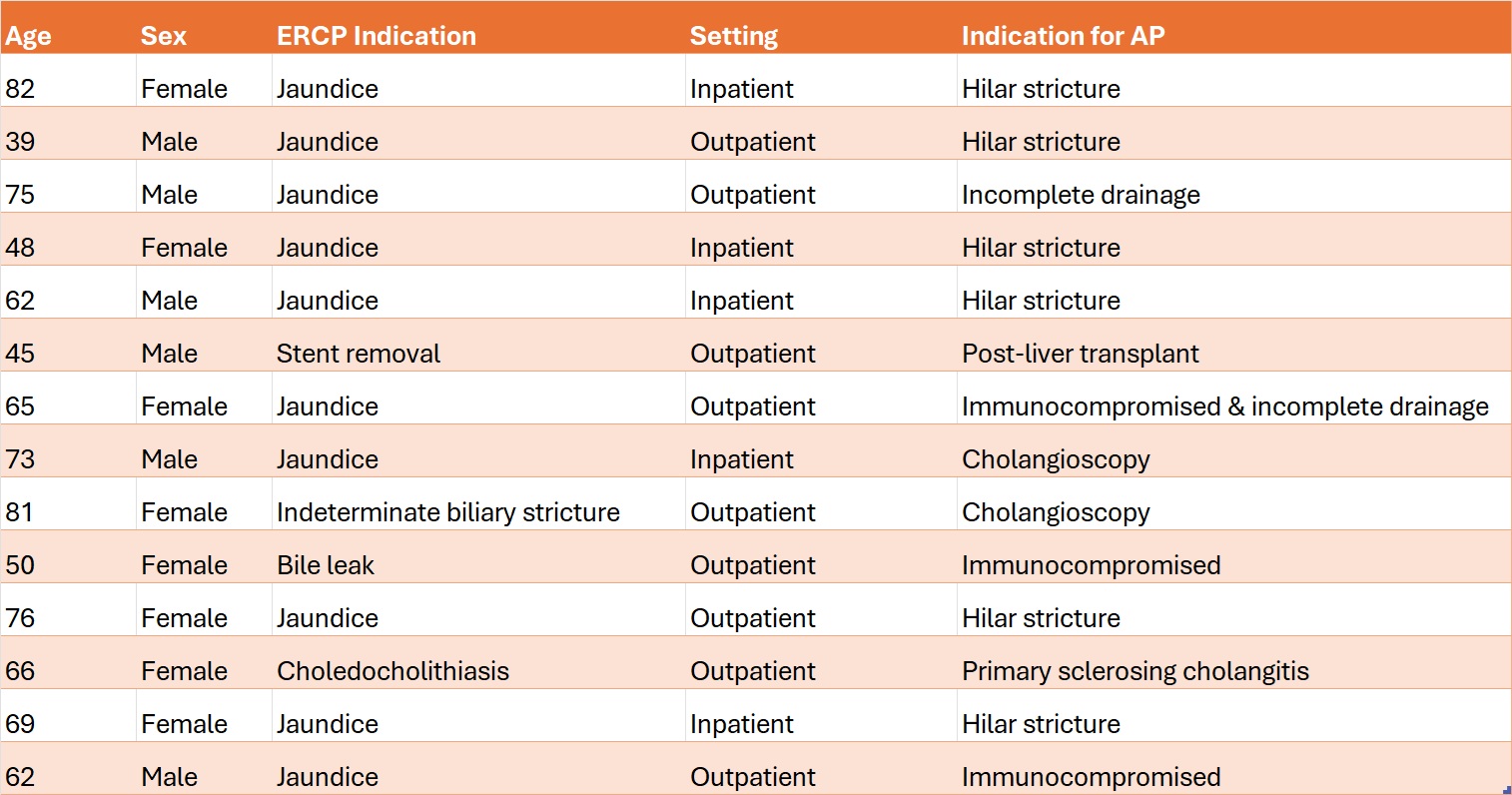
Figure: Table 2. Characteristics of post-ERCP infections with failed adherence to either ASGE or ESGE guidelines (n = 14)
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Sanjeevani Tomar indicated no relevant financial relationships.
Abdullah Abbasi indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Sagar Pathak indicated no relevant financial relationships.
Maham Hayat indicated no relevant financial relationships.
Deepanshu Jain indicated no relevant financial relationships.
Kambiz Kadkhodayan indicated no relevant financial relationships.
Dennis Yang: 3D-Matrix – Consultant. Apollo Endosurgery – Consultant. ERBE – Consultant. Fujifilm – Consultant. Medtronic – Consultant. MicroTech – Consultant. Olympus – Consultant.
Mustafa Arain: Boston Scientific – Consultant. Cook Endoscopy – Consultant. Olympus – Consultant.
Irteza Inayat indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. MicroTech Endoscopy – Consultant. Olympus America – Consultant.
Natalie Cosgrove indicated no relevant financial relationships.
Tareq Alsaleh, MD1, Sanjeevani Tomar, MD2, Abdullah Abbasi, MD3, Saurabh Chandan, MD3, Sagar J.. Pathak, MD3, Maham Hayat, MD3, Deepanshu Jain, MD3, Kambiz Kadkhodayan, MD3, Dennis Yang, MD, FACG4, Mustafa Arain, MD3, Irteza Inayat, MD5, Muhammad Hasan, MD, FACG3, Natalie Cosgrove, MD3. P3541 - Real World Assessment of Post-ERCP Infections From a Quality Dashboard, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, AdventHealth Orlando, Orlando, FL; 2Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 3Center for Interventional Endoscopy, AdventHealth Orlando, Orlando, FL; 4Center for Interventional Endoscopic, AdventHealth Orlando, Orlando, FL; 5Department of Gastroenterology and Hepatology, Adventhealth Orlando, Orlando, FL
Introduction: Endoscopic Retrograde Cholangiopancreatography (ERCP) is associated with risks including infections, particularly in high-risk scenarios. American Society for Gastrointestinal Endoscopy (ASGE) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines for ERCP antibiotic prophylaxis (AP) differ and are somewhat vague. Our center monitors ERCP outcomes using a Quality Dashboard. A review of post ERCP infections was performed to assess accuracy, guideline adherence, and identify areas for improvement.
Methods: Two quality data abstractors reviewed electronic medical records (EMRs) of all ERCP at a quaternary referral center between April 1, 2024, to May 22, 2025 as part of post ERCP quality outcome monitoring, and conducted follow-up calls at days 3-7 and 14-30 post procedure. Cases of suspected infection within 14 days of ERCP were assessed for adherence to guidelines and data collection accuracy.
Results: Abstractors noted 40 cases of post-ERCP infection out of 3,035 total ERCPs (1.32%). After physician review, abstractors were accurate in 36 out of 40 assessments (90%). Abstractor false positives included 3 pre-ERCP infections and 1 case of post-ERCP abdominal pain with SIRS.
In the 36 cases of true post-ERCP infection, AP was administered on the same day as ERCP in 10 procedures (27.8%). Among these, 3 (30%) received only peri-ERCP antibiotics, while 9 (90%) received antibiotics both peri-ERCP and for several days after. For 2 cases of true post-ERCP infection, patients were on antibiotics that were not considered AP (Daptomycin, Rifampin).
Most ERCPs with AP were inpatient procedures (7 out of 10, 70%), while most ERCPs without AP were outpatient procedures (17 out of 26, 65.4%) (Table 1). There was a lack of AP guideline adherence to either ASGE or ESGE guidelines in 14 cases of post-ERCP infections (38.9%). Most of those cases occurred in female patients and in the outpatient setting (Table 2).
Discussion: Post ERCP infection reporting by data abstractors was reasonably accurate, although false positives were noted. While infection rates were overall low, several cases were identified which may have been mitigated by following available guidelines, presenting opportunity for quality improvement. Additionally, infections attributed to post ERCP occurred in appropriately treated patients, reflecting potential limitations of the current guidelines. Enhanced guideline adherence, improved abstractor training, and refined risk stratification are warranted.

Figure: Table 1. Patient Characteristics for post-ERCP infections with and without antibiotic prophylaxis

Figure: Table 2. Characteristics of post-ERCP infections with failed adherence to either ASGE or ESGE guidelines (n = 14)
Disclosures:
Tareq Alsaleh indicated no relevant financial relationships.
Sanjeevani Tomar indicated no relevant financial relationships.
Abdullah Abbasi indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Sagar Pathak indicated no relevant financial relationships.
Maham Hayat indicated no relevant financial relationships.
Deepanshu Jain indicated no relevant financial relationships.
Kambiz Kadkhodayan indicated no relevant financial relationships.
Dennis Yang: 3D-Matrix – Consultant. Apollo Endosurgery – Consultant. ERBE – Consultant. Fujifilm – Consultant. Medtronic – Consultant. MicroTech – Consultant. Olympus – Consultant.
Mustafa Arain: Boston Scientific – Consultant. Cook Endoscopy – Consultant. Olympus – Consultant.
Irteza Inayat indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. MicroTech Endoscopy – Consultant. Olympus America – Consultant.
Natalie Cosgrove indicated no relevant financial relationships.
Tareq Alsaleh, MD1, Sanjeevani Tomar, MD2, Abdullah Abbasi, MD3, Saurabh Chandan, MD3, Sagar J.. Pathak, MD3, Maham Hayat, MD3, Deepanshu Jain, MD3, Kambiz Kadkhodayan, MD3, Dennis Yang, MD, FACG4, Mustafa Arain, MD3, Irteza Inayat, MD5, Muhammad Hasan, MD, FACG3, Natalie Cosgrove, MD3. P3541 - Real World Assessment of Post-ERCP Infections From a Quality Dashboard, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

