Monday Poster Session
Category: Interventional Endoscopy
P3537 - POSE-2 for Obesity: A Systematic Review and Meta-Analysis of Efficacy, Safety, and Metabolic Outcomes
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Umar Farooque, MBBS (he/him/his)
Health Education England, East of England, United Kingdom
Luton, England, United Kingdom
Presenting Author(s)
Umar Farooque, MBBS1, Syeda Hafsa Qadri, MBBS, MD2, Ana Beatriz Nardelli da Silva, MD3, Andela Malaj, MD, MPH4, Fnu Aparna, MBBS5, Meer Murtaza, MBBS6, Waseh Ahsan, MBBS, MD7, Arshia Warsi, 2, Aasma Shaukat, MD, MPH, FACG8
1Health Education England, East of England, United Kingdom, Luton, England, United Kingdom; 2Dow University of Health Sciences, Karachi, Sindh, Pakistan; 3Federal University of Pará, Belém, Para, Brazil; 4Vivantes Klinikum Kaulsdorf, Berlin, Berlin, Germany; 5Shaheed mohtarma benazir bhutto university, Karachi, Sindh, Pakistan; 6Liaquat University of Medical and Health Science, Shahpur Chakar, Sindh, Pakistan; 7Health Education Services Dothan Alabama, Gilgit, Northern Areas, Pakistan; 8NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Minimally invasive interventions for obesity are gaining prominence due to the limitations of bariatric surgery. POSE-2 (Primary Obesity Surgery Endoluminal 2) is a novel endoscopic bariatric procedure targeting gastric remodeling to promote weight loss. This study synthesizes the current evidence on POSE-2’s efficacy, safety, and metabolic effects.
Methods: A systematic review and meta-analysis was conducted following PRISMA guidelines. Databases including PubMed, Embase, and Cochrane were searched through January 2025. Eligible studies included randomized controlled trials (RCTs) and observational cohorts assessing POSE-2 outcomes in adults with obesity. Random-effects meta-analyses were performed to estimate pooled changes in total body weight loss percentage (TBWL%), excess weight loss percentage (EWL%), and metabolic markers (HbA1c, glucose, liver enzymes, serum cholesterol).
Results: Four studies (1 RCT, 3 cohorts; N = 210) were included. Pooled TBWL% progressively increased post-intervention: 13.23% (95% CI: 9.24–17.21%) at 3 months, 16.22% (95% CI: 12.83–19.61%) at 6 months, and 16.17% (95% CI: 14.11–18.23%) at 12 months (Figure 1). EWL% at 12 months was 56.95% (95% CI: 35.75–78.15%) (Figure 2). Significant reductions in HbA1c (SMD: –0.67; 95% CI: -1.15, -0.19) and serum cholesterol (SMD = -0.25; 95% CI: -0.32, -0.19) at 6 months were observed (Figure 2). No statistically significant changes were noted in fasting glucose or liver enzymes, though trends favored improvement. The overall adverse event rate was low and predominantly mild.
Discussion: POSE-2 achieves clinically meaningful and durable weight loss with minimal adverse events and potential glycemic benefit. While early results are encouraging, the limited evidence base necessitates larger, long-term RCTs. POSE-2 may emerge as a valuable tool in the endoscopic armamentarium for obesity management.
ChatGPT (OpenAI) was used for language clarity and grammar refinement. All scientific content, data, and analysis are original and solely by the authors.
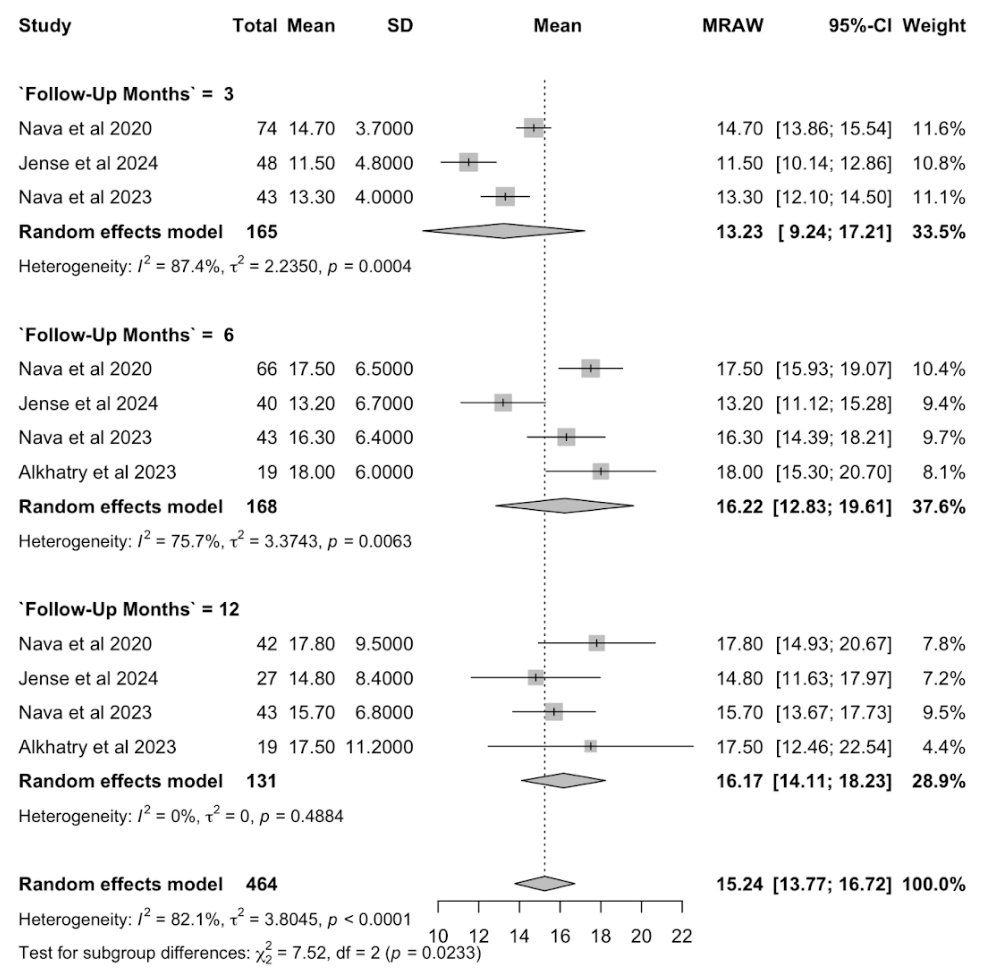
Figure: Figure 1. Forest plot of total body weight loss (%) at 3, 6, and 12 months of follow-up in adults undergoing POSE-2 intervention, using a random-effects model.
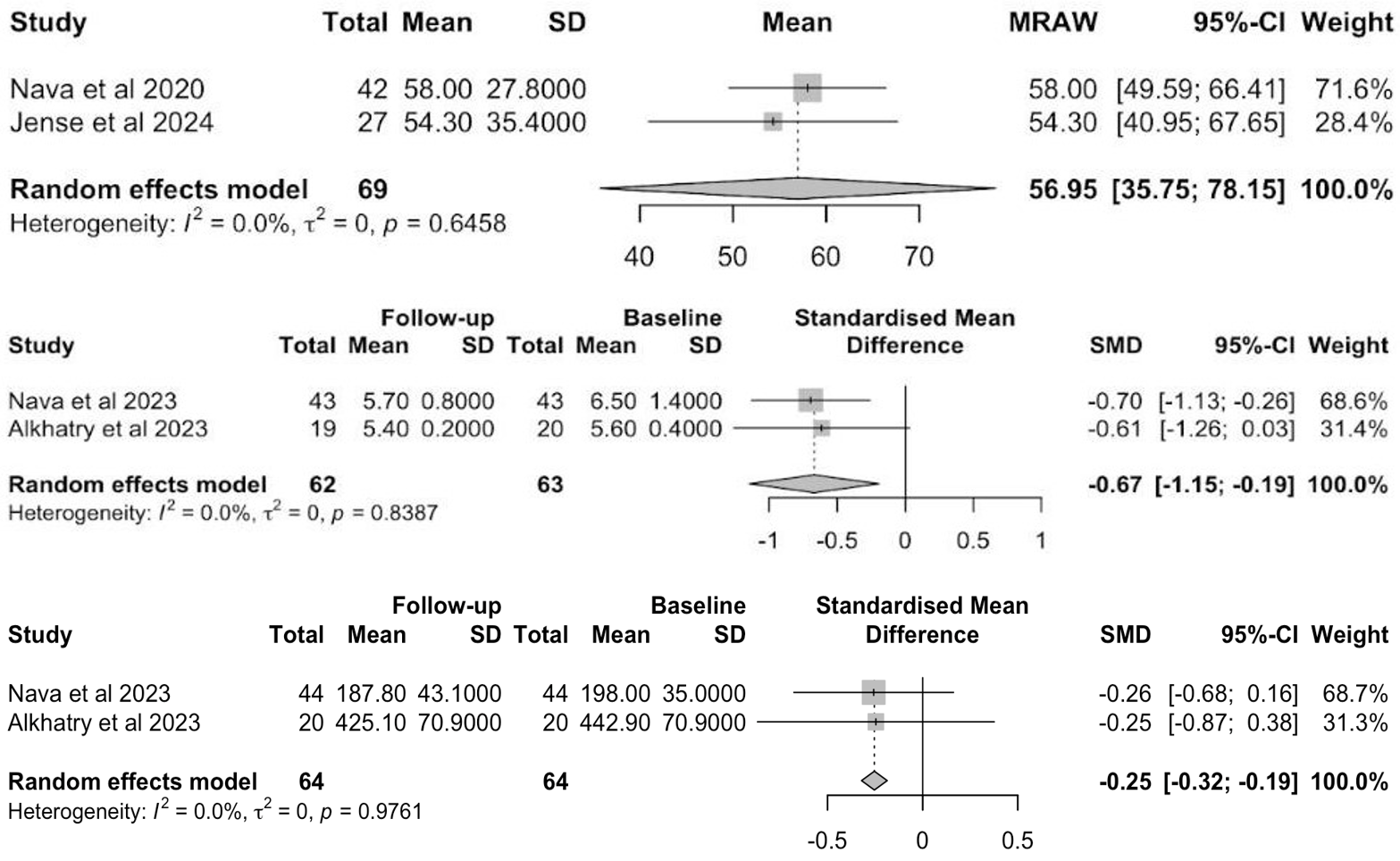
Figure: Figure 2. Forest plot of excess weight loss (%) at 12 months of follow-up (top), HbA1c (%) at 6 months of follow-up (middle), and serum cholesterol (mg/dL) at 6 months of follow-up (bottom) in adults undergoing POSE-2 intervention, using a random-effects model.
Disclosures:
Umar Farooque indicated no relevant financial relationships.
Syeda Hafsa Qadri indicated no relevant financial relationships.
Ana Beatriz Nardelli da Silva indicated no relevant financial relationships.
Andela Malaj indicated no relevant financial relationships.
Fnu Aparna indicated no relevant financial relationships.
Meer Murtaza indicated no relevant financial relationships.
Waseh Ahsan indicated no relevant financial relationships.
Arshia Warsi indicated no relevant financial relationships.
Aasma Shaukat: Freenome inc – Consultant.
Umar Farooque, MBBS1, Syeda Hafsa Qadri, MBBS, MD2, Ana Beatriz Nardelli da Silva, MD3, Andela Malaj, MD, MPH4, Fnu Aparna, MBBS5, Meer Murtaza, MBBS6, Waseh Ahsan, MBBS, MD7, Arshia Warsi, 2, Aasma Shaukat, MD, MPH, FACG8. P3537 - POSE-2 for Obesity: A Systematic Review and Meta-Analysis of Efficacy, Safety, and Metabolic Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Health Education England, East of England, United Kingdom, Luton, England, United Kingdom; 2Dow University of Health Sciences, Karachi, Sindh, Pakistan; 3Federal University of Pará, Belém, Para, Brazil; 4Vivantes Klinikum Kaulsdorf, Berlin, Berlin, Germany; 5Shaheed mohtarma benazir bhutto university, Karachi, Sindh, Pakistan; 6Liaquat University of Medical and Health Science, Shahpur Chakar, Sindh, Pakistan; 7Health Education Services Dothan Alabama, Gilgit, Northern Areas, Pakistan; 8NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Minimally invasive interventions for obesity are gaining prominence due to the limitations of bariatric surgery. POSE-2 (Primary Obesity Surgery Endoluminal 2) is a novel endoscopic bariatric procedure targeting gastric remodeling to promote weight loss. This study synthesizes the current evidence on POSE-2’s efficacy, safety, and metabolic effects.
Methods: A systematic review and meta-analysis was conducted following PRISMA guidelines. Databases including PubMed, Embase, and Cochrane were searched through January 2025. Eligible studies included randomized controlled trials (RCTs) and observational cohorts assessing POSE-2 outcomes in adults with obesity. Random-effects meta-analyses were performed to estimate pooled changes in total body weight loss percentage (TBWL%), excess weight loss percentage (EWL%), and metabolic markers (HbA1c, glucose, liver enzymes, serum cholesterol).
Results: Four studies (1 RCT, 3 cohorts; N = 210) were included. Pooled TBWL% progressively increased post-intervention: 13.23% (95% CI: 9.24–17.21%) at 3 months, 16.22% (95% CI: 12.83–19.61%) at 6 months, and 16.17% (95% CI: 14.11–18.23%) at 12 months (Figure 1). EWL% at 12 months was 56.95% (95% CI: 35.75–78.15%) (Figure 2). Significant reductions in HbA1c (SMD: –0.67; 95% CI: -1.15, -0.19) and serum cholesterol (SMD = -0.25; 95% CI: -0.32, -0.19) at 6 months were observed (Figure 2). No statistically significant changes were noted in fasting glucose or liver enzymes, though trends favored improvement. The overall adverse event rate was low and predominantly mild.
Discussion: POSE-2 achieves clinically meaningful and durable weight loss with minimal adverse events and potential glycemic benefit. While early results are encouraging, the limited evidence base necessitates larger, long-term RCTs. POSE-2 may emerge as a valuable tool in the endoscopic armamentarium for obesity management.
ChatGPT (OpenAI) was used for language clarity and grammar refinement. All scientific content, data, and analysis are original and solely by the authors.

Figure: Figure 1. Forest plot of total body weight loss (%) at 3, 6, and 12 months of follow-up in adults undergoing POSE-2 intervention, using a random-effects model.

Figure: Figure 2. Forest plot of excess weight loss (%) at 12 months of follow-up (top), HbA1c (%) at 6 months of follow-up (middle), and serum cholesterol (mg/dL) at 6 months of follow-up (bottom) in adults undergoing POSE-2 intervention, using a random-effects model.
Disclosures:
Umar Farooque indicated no relevant financial relationships.
Syeda Hafsa Qadri indicated no relevant financial relationships.
Ana Beatriz Nardelli da Silva indicated no relevant financial relationships.
Andela Malaj indicated no relevant financial relationships.
Fnu Aparna indicated no relevant financial relationships.
Meer Murtaza indicated no relevant financial relationships.
Waseh Ahsan indicated no relevant financial relationships.
Arshia Warsi indicated no relevant financial relationships.
Aasma Shaukat: Freenome inc – Consultant.
Umar Farooque, MBBS1, Syeda Hafsa Qadri, MBBS, MD2, Ana Beatriz Nardelli da Silva, MD3, Andela Malaj, MD, MPH4, Fnu Aparna, MBBS5, Meer Murtaza, MBBS6, Waseh Ahsan, MBBS, MD7, Arshia Warsi, 2, Aasma Shaukat, MD, MPH, FACG8. P3537 - POSE-2 for Obesity: A Systematic Review and Meta-Analysis of Efficacy, Safety, and Metabolic Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
