Monday Poster Session
Category: IBD
P3380 - Case Report: Management of Upadacitinib Induced Anemia in a Patient With Ulcerative Colitis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aneri Vakharia, MD, MS (she/her/hers)
Northwell Health
Manhasset, NY
Presenting Author(s)
Aneri Vakharia, MD, MS1, Keith Sultan, MD, FACG2, Michael Delicce, MD1
1Northwell Health, Manhasset, NY; 2Zucker School of Medicine Hofstra University, Hempstead, NY
Introduction: Upadacitinib (UPA) is a Janus Kinase (JAK) inhibitor currently approved for moderate to severe ulcerative colitis (UC). Drug-induced anemia with hemoglobin (hgb) levels less than 8 grams per deciliter (g/dL) is an adverse event that has been observed in JAK clinical trials.1 The current prescribing recommendation for those who experience anemia related to UPA is to stop treatment, noting that the anemia is generally reversible. However, there is little known about the safety and success of resuming UPA in such patients. We present a case of UPA-associated anemia in a patient with UC who achieved successful re-initiation of therapy without recurrent anemia while maintaining clinical remission.
Case Description/
Methods: A 55-year-old female with moderate to severe UC started on UPA 45 milligrams (mg) daily after failure to control her disease with adalimumab and infliximab. Despite a rapid improvement of bowel habit, within one month her hgb dropped from 13.2 g/dl to 8.7 g/dl. UPA was held and workup revealed normal iron studies, normal upper endoscopy and video capsule endoscopy, along with a colonoscopy showing improved colitis with no bleeding source. Further work-up by hematology revealed no alternative etiology. As the patient’s bowel complaints resumed and the hgb improved to 10.7 g/dl, UPA was re-initiated at 30mg daily. Several weeks after resuming UPA the patient recaptured clinical remission, her hgb rose to 12.0 g/dl and remains normal at 3-month follow-up.
Discussion: UC is a debilitating disease with significant morbidity. While several therapies exist for treatment of UC, UPA is among the most effective.2 As anemia is one of the most reported adverse events related to UPA, it is imperative to develop an effective management strategy that balances the needs of medication efficacy and safety. Our case demonstrates a patient whose severe anemia was successfully corrected, then maintained with resumption of UPA at a lower dose while achieving a successful response to therapy. Our case suggests that patients who develop drug-induced anemia on induction dosing of UPA can be considered for re-initiation of therapy at a lower dose following recovery of hgb in the appropriate clinical setting.
Evaluating Upadacitinib in the Treatment of Moderate-to-Severe Active Ulcerative Colitis. Napolitano M, et al. Drug Design, Development and Therapy. 2022
Ananthakrishnan AN, et al. Comparative Efficacy of Advanced Therapies for Management of Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2024
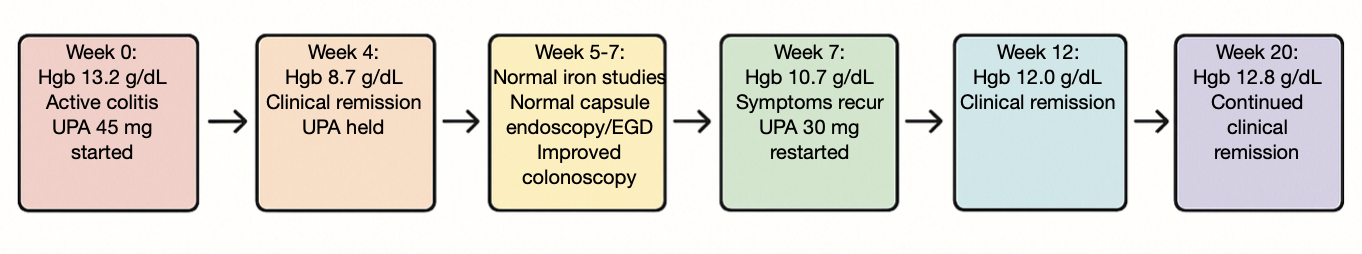
Figure: Upadacitinib (UPA) Treatment Timeline (partially generated through use of AI)
Disclosures:
Aneri Vakharia indicated no relevant financial relationships.
Keith Sultan indicated no relevant financial relationships.
Michael Delicce indicated no relevant financial relationships.
Aneri Vakharia, MD, MS1, Keith Sultan, MD, FACG2, Michael Delicce, MD1. P3380 -
Case Report: Management of Upadacitinib Induced Anemia in a Patient With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Northwell Health, Manhasset, NY; 2Zucker School of Medicine Hofstra University, Hempstead, NY
Introduction: Upadacitinib (UPA) is a Janus Kinase (JAK) inhibitor currently approved for moderate to severe ulcerative colitis (UC). Drug-induced anemia with hemoglobin (hgb) levels less than 8 grams per deciliter (g/dL) is an adverse event that has been observed in JAK clinical trials.1 The current prescribing recommendation for those who experience anemia related to UPA is to stop treatment, noting that the anemia is generally reversible. However, there is little known about the safety and success of resuming UPA in such patients. We present a case of UPA-associated anemia in a patient with UC who achieved successful re-initiation of therapy without recurrent anemia while maintaining clinical remission.
Case Description/
Methods: A 55-year-old female with moderate to severe UC started on UPA 45 milligrams (mg) daily after failure to control her disease with adalimumab and infliximab. Despite a rapid improvement of bowel habit, within one month her hgb dropped from 13.2 g/dl to 8.7 g/dl. UPA was held and workup revealed normal iron studies, normal upper endoscopy and video capsule endoscopy, along with a colonoscopy showing improved colitis with no bleeding source. Further work-up by hematology revealed no alternative etiology. As the patient’s bowel complaints resumed and the hgb improved to 10.7 g/dl, UPA was re-initiated at 30mg daily. Several weeks after resuming UPA the patient recaptured clinical remission, her hgb rose to 12.0 g/dl and remains normal at 3-month follow-up.
Discussion: UC is a debilitating disease with significant morbidity. While several therapies exist for treatment of UC, UPA is among the most effective.2 As anemia is one of the most reported adverse events related to UPA, it is imperative to develop an effective management strategy that balances the needs of medication efficacy and safety. Our case demonstrates a patient whose severe anemia was successfully corrected, then maintained with resumption of UPA at a lower dose while achieving a successful response to therapy. Our case suggests that patients who develop drug-induced anemia on induction dosing of UPA can be considered for re-initiation of therapy at a lower dose following recovery of hgb in the appropriate clinical setting.
Evaluating Upadacitinib in the Treatment of Moderate-to-Severe Active Ulcerative Colitis. Napolitano M, et al. Drug Design, Development and Therapy. 2022
Ananthakrishnan AN, et al. Comparative Efficacy of Advanced Therapies for Management of Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2024

Figure: Upadacitinib (UPA) Treatment Timeline (partially generated through use of AI)
Disclosures:
Aneri Vakharia indicated no relevant financial relationships.
Keith Sultan indicated no relevant financial relationships.
Michael Delicce indicated no relevant financial relationships.
Aneri Vakharia, MD, MS1, Keith Sultan, MD, FACG2, Michael Delicce, MD1. P3380 -
Case Report: Management of Upadacitinib Induced Anemia in a Patient With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
