Monday Poster Session
Category: IBD
P3333 - Characterizing Indicators of Suboptimal Treatment With Conventional Therapies for Ulcerative Colitis (UC): A Systematic Literature Review
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jordan Axelrad, MD, MPH
Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine
New York, NY
Presenting Author(s)
Jordan Axelrad, MD, MPH1, Karolina Wosik, MSc, PhD2, Raju Gautam, PhD3, Ratna Pandey, MSc4, Christina Cognata, PharmD, MBA5, Joseph C. Cappelleri, PhD, MPH6, Peter Hur, PharmD7, Shameer Mehta, MD8
1Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 2Pfizer Canada, Kirkland, PQ, Canada; 3ConnectHEOR, London, England, United Kingdom; 4ConnectHEOR, New Delhi, Delhi, India; 5Pfizer Inc, Collegeville, PA; 6Pfizer Inc, Groton, CT; 7Pfizer Inc, New York, NY; 8The Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom
Introduction: Synthesis of evidence on rates, definitions and outcomes of indicators of suboptimal treatment with conventional therapies (CTs) in patients with UC are needed to clarify interpretation of study outcomes, inform future study designs, and develop predictive risk tools for early identification of suboptimal treatment with CT for treatment escalation and optimization.
Methods: A systematic literature review following Cochrane Collaboration and PRISMA guidelines was conducted for studies assessing definitions and rates of indicators of suboptimal treatment and disease burden with CTs (5-ASAs, corticosteroids, immunomodulators). It included full papers (Jan 2014–Nov 2024) and conference abstracts (Jan 2022–Nov 2024) published in English for observational cohort studies and post-hoc analyses of clinical trials of adult patients with UC.
Results: Overall, 78 studies were included (Europe, 45%; US, 21%) using medical records (69%), claims (18%), registries (8%) and surveys (5%). Among the 61 studies with indicators of suboptimal treatment with CTs, 5% explicitly defined ‘suboptimal’ for assessment of CTs, 39% defined ≥1 suboptimal indicator, and 56% had undefined suboptimal indicators (Figure 1). Definitions and rates of suboptimal indicators are shown in Table 1, where many patients had an indicator of ‘suboptimal’ treatment, discontinuation, relapse, and non-response. Given differences in study designs and indicator definitions, findings may not be comparable across studies.
Disease burden associated with indicators of CT suboptimal treatment was high with nearly double the rate of inpatient visits and higher medical and drug costs compared with those without suboptimal indicators. Studies showed that few patients transitioned to an advanced therapy (AT) including 5.9% switching to AT in a US study (mean follow-up, 2.3 years) and low biologic use in the 6th line (14.2%; mean follow-up, 3.2 years) in a Japanese study. Four studies assessed predictive risk factors of indicators of 5-ASA suboptimal treatment, with greater disease extent and higher disease activity as common significant predictors.
Discussion: Although definitions and study designs varied, many patients with UC receiving CTs had an indicator of suboptimal treatment and higher disease burden and costs. Few patients transitioned to an AT over the study periods, suggesting a need for validated predictive models or clinical scoring tools that may aid with appropriate escalation of therapy.
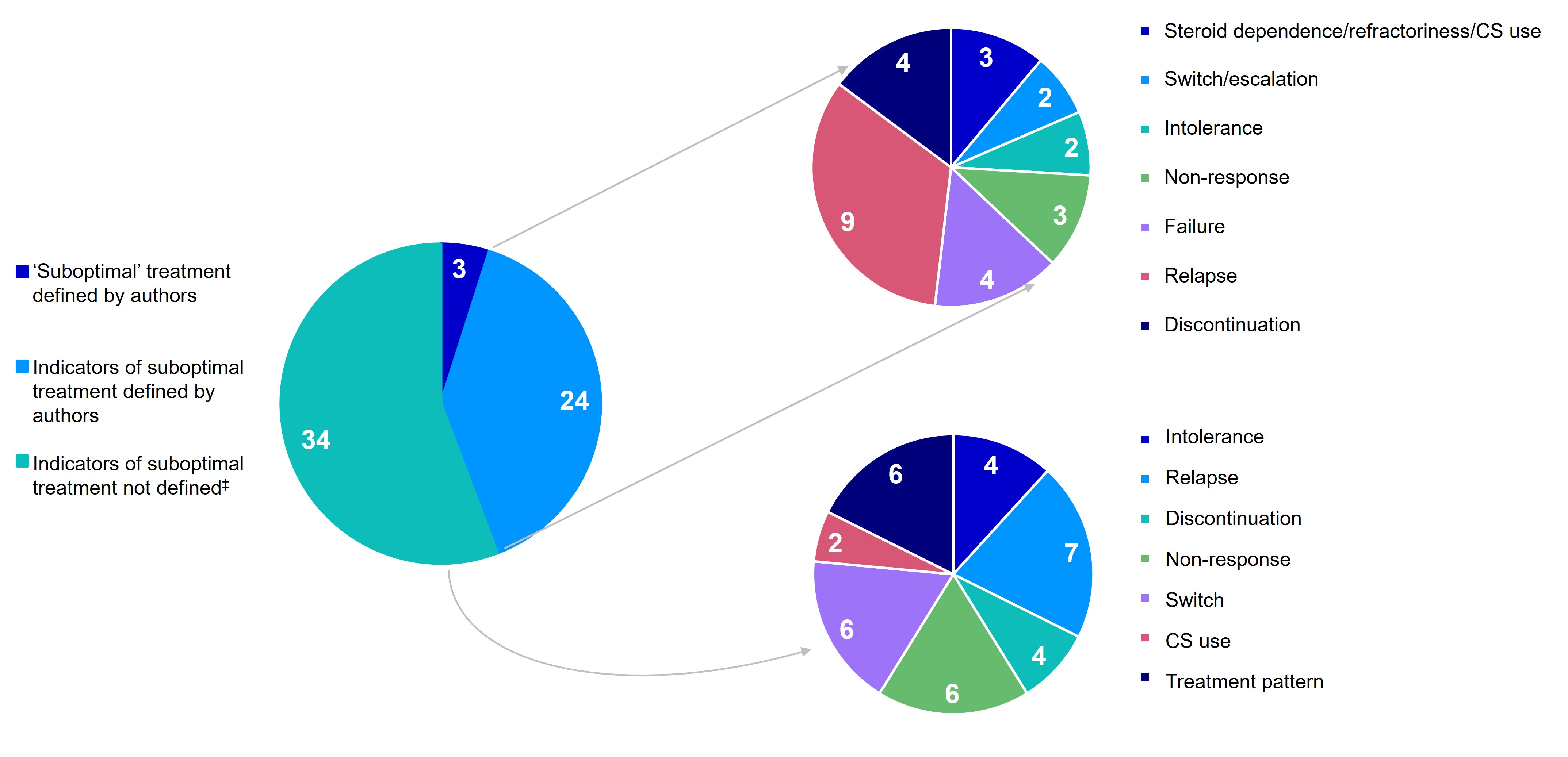
Figure: Figure 1. Distribution of studies* reporting indicators of suboptimal treatment with CTs and definitions† if available.
CS, corticosteroid; CT, conventional therapy. *Data points are reported as number of studies; some studies include multiple indicators of suboptimal treatment with CTs. †Single indicators of suboptimal treatments with CTs that were components of explicitly defined ‘suboptimal’ treatment composite outcomes are not represented in the pie charts illustrating the different indicators of suboptimal treatment with CTs. ‡Indicators of suboptimal treatment with CTs were included in the study but not explicitly defined by the authors.

Figure: Table 1. Definitions and rates of indicators of suboptimal treatment with CTs that were supported by multiple studies with a follow-up period of >6 months.
AE, adverse event; ASA, aminosalicylic acid; AT, advanced therapy; AZA, azathioprine; CPT, Current Procedural Terminology; CS, corticosteroid; CT, conventional therapy; EIM, extraintestinal manifestations; GI, gastrointestinal; IBD, irritable bowel disease; IMM, immunomodulator, NR, not reported; RBS, rectal bleeding score; TGN, thioguanine nucleotide; TNF, tumor necrosis factor; UC, ulcerative colitis; UC-DAI, ulcerative colitis disease activity index.
*Thiopurine includes AZA, mercaptopurine, and thioguanine.
Disclosures:
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
Raju Gautam: ConnectHEOR – Employee.
Ratna Pandey: ConnectHEOR – Employee.
Christina Cognata: Pfizer Inc – Employee, Stock Options.
Joseph Cappelleri: Pfizer Inc – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Shameer Mehta: AbbVie – Speakers Bureau. B Braun and Baxter – Grant/Research Support. Johnson & Johnson – Advisor or Review Panel Member. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Takeda – Grant/Research Support, Speakers Bureau.
Jordan Axelrad, MD, MPH1, Karolina Wosik, MSc, PhD2, Raju Gautam, PhD3, Ratna Pandey, MSc4, Christina Cognata, PharmD, MBA5, Joseph C. Cappelleri, PhD, MPH6, Peter Hur, PharmD7, Shameer Mehta, MD8. P3333 - Characterizing Indicators of Suboptimal Treatment With Conventional Therapies for Ulcerative Colitis (UC): A Systematic Literature Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 2Pfizer Canada, Kirkland, PQ, Canada; 3ConnectHEOR, London, England, United Kingdom; 4ConnectHEOR, New Delhi, Delhi, India; 5Pfizer Inc, Collegeville, PA; 6Pfizer Inc, Groton, CT; 7Pfizer Inc, New York, NY; 8The Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom
Introduction: Synthesis of evidence on rates, definitions and outcomes of indicators of suboptimal treatment with conventional therapies (CTs) in patients with UC are needed to clarify interpretation of study outcomes, inform future study designs, and develop predictive risk tools for early identification of suboptimal treatment with CT for treatment escalation and optimization.
Methods: A systematic literature review following Cochrane Collaboration and PRISMA guidelines was conducted for studies assessing definitions and rates of indicators of suboptimal treatment and disease burden with CTs (5-ASAs, corticosteroids, immunomodulators). It included full papers (Jan 2014–Nov 2024) and conference abstracts (Jan 2022–Nov 2024) published in English for observational cohort studies and post-hoc analyses of clinical trials of adult patients with UC.
Results: Overall, 78 studies were included (Europe, 45%; US, 21%) using medical records (69%), claims (18%), registries (8%) and surveys (5%). Among the 61 studies with indicators of suboptimal treatment with CTs, 5% explicitly defined ‘suboptimal’ for assessment of CTs, 39% defined ≥1 suboptimal indicator, and 56% had undefined suboptimal indicators (Figure 1). Definitions and rates of suboptimal indicators are shown in Table 1, where many patients had an indicator of ‘suboptimal’ treatment, discontinuation, relapse, and non-response. Given differences in study designs and indicator definitions, findings may not be comparable across studies.
Disease burden associated with indicators of CT suboptimal treatment was high with nearly double the rate of inpatient visits and higher medical and drug costs compared with those without suboptimal indicators. Studies showed that few patients transitioned to an advanced therapy (AT) including 5.9% switching to AT in a US study (mean follow-up, 2.3 years) and low biologic use in the 6th line (14.2%; mean follow-up, 3.2 years) in a Japanese study. Four studies assessed predictive risk factors of indicators of 5-ASA suboptimal treatment, with greater disease extent and higher disease activity as common significant predictors.
Discussion: Although definitions and study designs varied, many patients with UC receiving CTs had an indicator of suboptimal treatment and higher disease burden and costs. Few patients transitioned to an AT over the study periods, suggesting a need for validated predictive models or clinical scoring tools that may aid with appropriate escalation of therapy.

Figure: Figure 1. Distribution of studies* reporting indicators of suboptimal treatment with CTs and definitions† if available.
CS, corticosteroid; CT, conventional therapy. *Data points are reported as number of studies; some studies include multiple indicators of suboptimal treatment with CTs. †Single indicators of suboptimal treatments with CTs that were components of explicitly defined ‘suboptimal’ treatment composite outcomes are not represented in the pie charts illustrating the different indicators of suboptimal treatment with CTs. ‡Indicators of suboptimal treatment with CTs were included in the study but not explicitly defined by the authors.

Figure: Table 1. Definitions and rates of indicators of suboptimal treatment with CTs that were supported by multiple studies with a follow-up period of >6 months.
AE, adverse event; ASA, aminosalicylic acid; AT, advanced therapy; AZA, azathioprine; CPT, Current Procedural Terminology; CS, corticosteroid; CT, conventional therapy; EIM, extraintestinal manifestations; GI, gastrointestinal; IBD, irritable bowel disease; IMM, immunomodulator, NR, not reported; RBS, rectal bleeding score; TGN, thioguanine nucleotide; TNF, tumor necrosis factor; UC, ulcerative colitis; UC-DAI, ulcerative colitis disease activity index.
*Thiopurine includes AZA, mercaptopurine, and thioguanine.
Disclosures:
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Karolina Wosik: Pfizer Canada Inc – Employee. Pfizer Inc – Stock Options.
Raju Gautam: ConnectHEOR – Employee.
Ratna Pandey: ConnectHEOR – Employee.
Christina Cognata: Pfizer Inc – Employee, Stock Options.
Joseph Cappelleri: Pfizer Inc – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Shameer Mehta: AbbVie – Speakers Bureau. B Braun and Baxter – Grant/Research Support. Johnson & Johnson – Advisor or Review Panel Member. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Takeda – Grant/Research Support, Speakers Bureau.
Jordan Axelrad, MD, MPH1, Karolina Wosik, MSc, PhD2, Raju Gautam, PhD3, Ratna Pandey, MSc4, Christina Cognata, PharmD, MBA5, Joseph C. Cappelleri, PhD, MPH6, Peter Hur, PharmD7, Shameer Mehta, MD8. P3333 - Characterizing Indicators of Suboptimal Treatment With Conventional Therapies for Ulcerative Colitis (UC): A Systematic Literature Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
