Monday Poster Session
Category: IBD
P3332 - Predictors of Response to Ustekinumab and Identification of Factors Associated With Dose Escalation in Patients With Inflammatory Bowel Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jordan Axelrad, MD, MPH
Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine
New York, NY
Presenting Author(s)
Jordan Axelrad, MD, MPH1, Sara Horst, MD, MPH, FACG2, Audrey Bennett, MD2, Anagha Ashokan, BS2, Dania Hudhud, MD3, Angela Wu, MD4, Millie D. Long, MD, FACG5, Chelsea Anderson, PhD, MPH6, Vasantham Chaudhary, MD7, Ellen M. Axenfeld, MD8, Raymond K. Cross, MD, MS, FACG9
1Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 2Vanderbilt University School of Medicine, Nashville, TN; 3University of Maryland School of Medicine, Baltimore, MD; 4MedStar Georgetown University Hospital, Washington, DC; 5Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 6UNC School of Medicine, Chapel Hill, NC; 7NYU Grossman School of Medicine, New York, NY; 8NYU Langone Health Inflammatory Bowel Disease Center, New York, NY; 9The Melissa L. Posner Institute for Digestive Health & Liver Disease, Mercy Medical Center, Baltimore, MD
Introduction: Dose escalation of ustekinumab for inflammatory bowel disease (IBD) is common. We aimed to determine the proportion of patients with Crohn’s disease (CD) and ulcerative colitis (UC) who required dose escalation after intravenous induction, and to identify clinical factors associated with escalation.
Methods: We performed a multicenter retrospective cohort study of adults with IBD initiating ustekinumab from 2016–2022 with ≥1 year of follow-up across five tertiary centers. The primary outcome was dose escalation, defined as shortening the maintenance interval and/or additional IV induction. Patients were stratified by IBD subtype and prior advanced therapy (AT) exposure. Multivariable regression identified factors associated with dose escalation and remission.
Results: We identified 1,202 patients initiating ustekinumab (58% female, mean age 41.7 years), 80% with CD, 18% UC, and 13% AT–naïve (Table 1). In CD, ileocolonic disease (58%), inflammatory phenotype (50%), perianal involvement (26%), and prior surgery (52%) were common. AT–exposed patients had higher rates of prior surgery (55% vs. 28%) and perianal disease (28% vs. 11%) than AT–naïve patients. In UC, 61% had pancolitis. At ustekinumab initiation, inflammatory markers (mean CRP 10.3 mg/L, fecal calprotectin 432.6 μg/g) were elevated and 42% were on corticosteroids. Within one year, 52% required escalation—more frequently in AT–exposed patients (54%) than AT–naïve (39%, Table 2). Most escalated to q6wks (53%) or q4wks (46%); repeat IV induction was rare (6%), especially in AT–naïve (1%). Common reasons for escalation included persistent symptoms (52%), low drug levels (56%), and endoscopic activity (25%). At 12 months, 960 patients remained on ustekinumab, with higher persistence in AT–naïve patients (88% vs. 83%). Clinical remission was achieved in 70% of AT–naïve and 57% of AT–exposed patients. Among those with endoscopic data, mucosal healing was more frequent in AT–naïve (68%) vs. AT–exposed (41%). Dose escalation was independently associated with prior AT exposure (aOR 2.00, 95% CI 1.34–2.99) and UC/IBD-U subtype (aOR 1.81, 95% CI 1.26–2.61). In CD, AT exposure and higher weight were linked to escalation; in UC/IBD-U, higher weight was associated.
Discussion: Over half with IBD initiating ustekinumab required dose escalation, particularly those with prior AT exposure, UC, or higher weight. Despite this, ustekinumab was associated with high treatment persistence and favorable clinical and endoscopic outcomes.
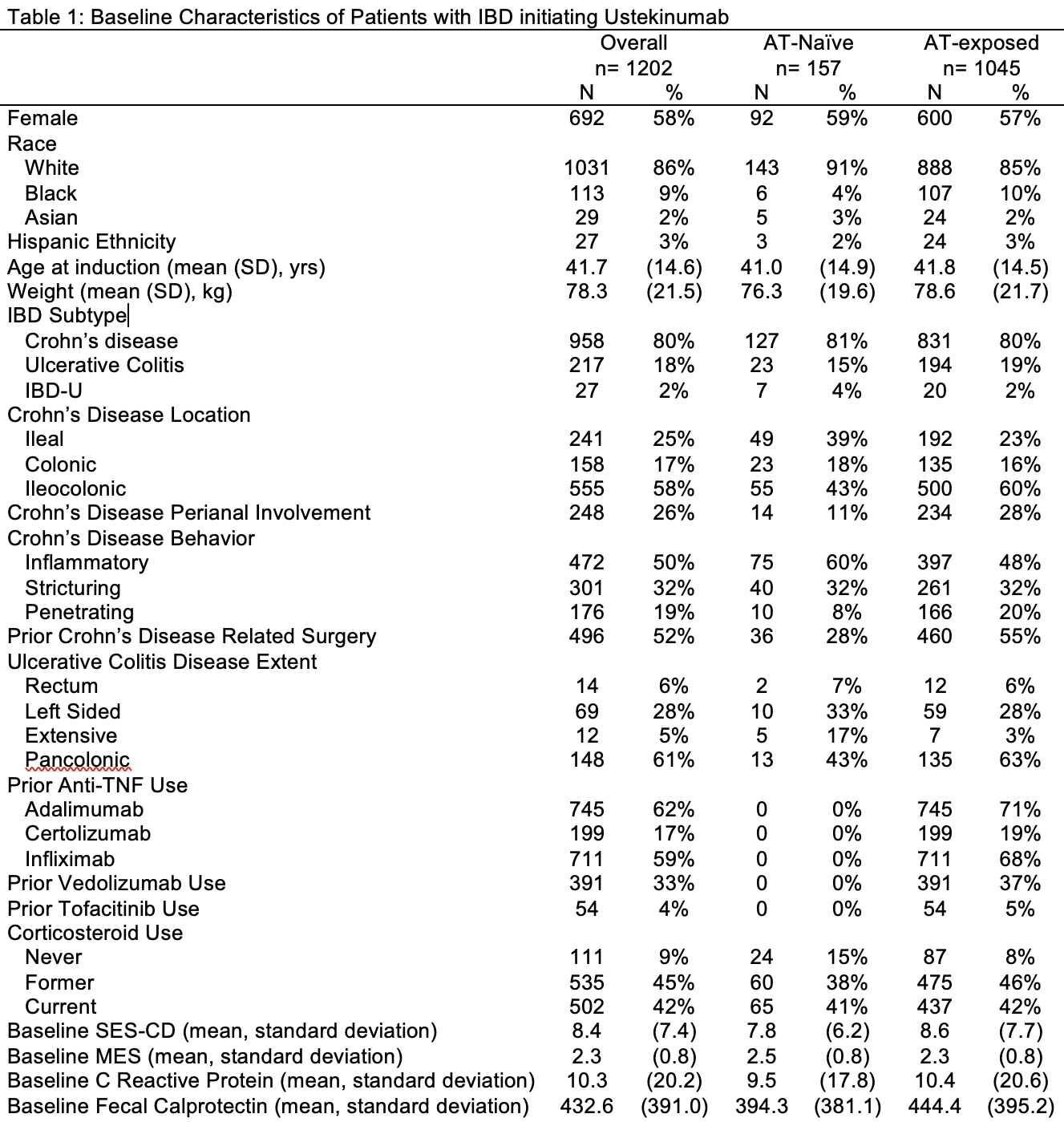
Figure: Table 1. Baseline Characteristics of Patients with IBD initiating Ustekinumab.
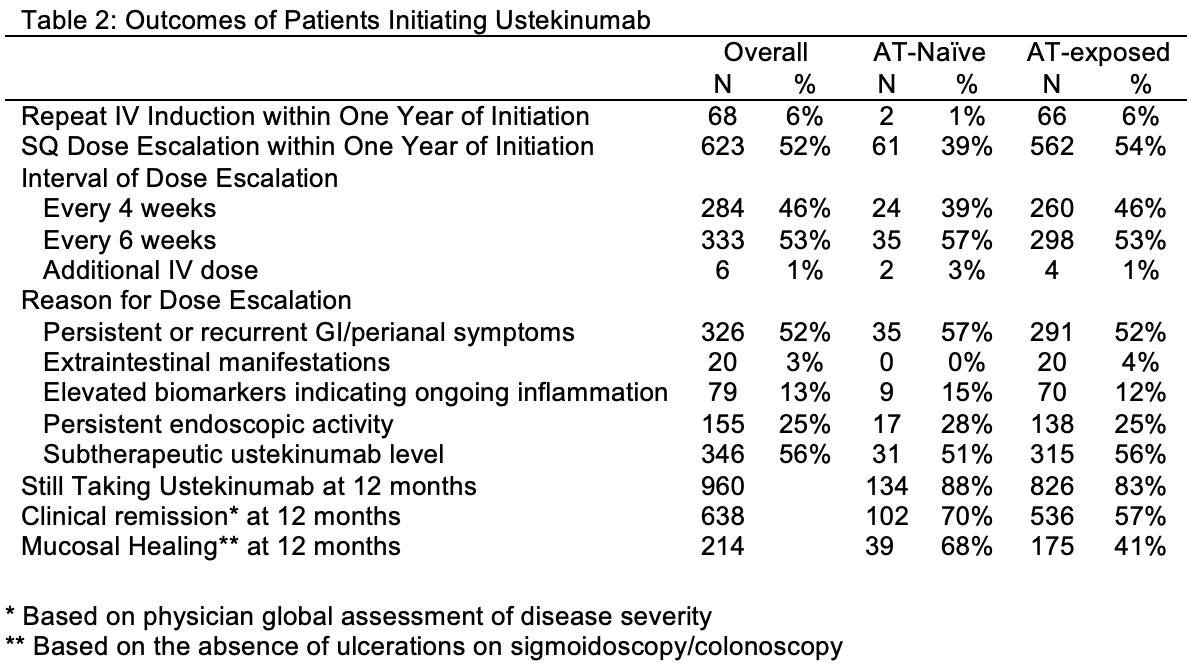
Figure: Table 2. Outcomes of Patients Initiating Ustekinumab.
Disclosures:
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Sara Horst: Abbvie – Consultant. Biocon – Consultant. Celltrion – Consultant. J&J – Consultant. Pfizer – Consultant. Takeda – Consultant.
Audrey Bennett: Celltrion – Consultant. Pfizer – Grant/Research Support.
Anagha Ashokan indicated no relevant financial relationships.
Dania Hudhud indicated no relevant financial relationships.
Angela Wu indicated no relevant financial relationships.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Chelsea Anderson indicated no relevant financial relationships.
Vasantham Chaudhary indicated no relevant financial relationships.
Ellen Axenfeld indicated no relevant financial relationships.
Raymond K. Cross: Abbvie – Advisor or Review Panel Member, Consultant, Speakers Bureau. BMS – Advisor or Review Panel Member, Consultant, Speakers Bureau. CorEvitas – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member, Consultant. Genetech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. IBD Education Group – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Advisory Committee/Board Member, Consultant. Option Care – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member, Consultant. Samsung Bioepis – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Takeda – Grant/Research Support.
Jordan Axelrad, MD, MPH1, Sara Horst, MD, MPH, FACG2, Audrey Bennett, MD2, Anagha Ashokan, BS2, Dania Hudhud, MD3, Angela Wu, MD4, Millie D. Long, MD, FACG5, Chelsea Anderson, PhD, MPH6, Vasantham Chaudhary, MD7, Ellen M. Axenfeld, MD8, Raymond K. Cross, MD, MS, FACG9. P3332 - Predictors of Response to Ustekinumab and Identification of Factors Associated With Dose Escalation in Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; 2Vanderbilt University School of Medicine, Nashville, TN; 3University of Maryland School of Medicine, Baltimore, MD; 4MedStar Georgetown University Hospital, Washington, DC; 5Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 6UNC School of Medicine, Chapel Hill, NC; 7NYU Grossman School of Medicine, New York, NY; 8NYU Langone Health Inflammatory Bowel Disease Center, New York, NY; 9The Melissa L. Posner Institute for Digestive Health & Liver Disease, Mercy Medical Center, Baltimore, MD
Introduction: Dose escalation of ustekinumab for inflammatory bowel disease (IBD) is common. We aimed to determine the proportion of patients with Crohn’s disease (CD) and ulcerative colitis (UC) who required dose escalation after intravenous induction, and to identify clinical factors associated with escalation.
Methods: We performed a multicenter retrospective cohort study of adults with IBD initiating ustekinumab from 2016–2022 with ≥1 year of follow-up across five tertiary centers. The primary outcome was dose escalation, defined as shortening the maintenance interval and/or additional IV induction. Patients were stratified by IBD subtype and prior advanced therapy (AT) exposure. Multivariable regression identified factors associated with dose escalation and remission.
Results: We identified 1,202 patients initiating ustekinumab (58% female, mean age 41.7 years), 80% with CD, 18% UC, and 13% AT–naïve (Table 1). In CD, ileocolonic disease (58%), inflammatory phenotype (50%), perianal involvement (26%), and prior surgery (52%) were common. AT–exposed patients had higher rates of prior surgery (55% vs. 28%) and perianal disease (28% vs. 11%) than AT–naïve patients. In UC, 61% had pancolitis. At ustekinumab initiation, inflammatory markers (mean CRP 10.3 mg/L, fecal calprotectin 432.6 μg/g) were elevated and 42% were on corticosteroids. Within one year, 52% required escalation—more frequently in AT–exposed patients (54%) than AT–naïve (39%, Table 2). Most escalated to q6wks (53%) or q4wks (46%); repeat IV induction was rare (6%), especially in AT–naïve (1%). Common reasons for escalation included persistent symptoms (52%), low drug levels (56%), and endoscopic activity (25%). At 12 months, 960 patients remained on ustekinumab, with higher persistence in AT–naïve patients (88% vs. 83%). Clinical remission was achieved in 70% of AT–naïve and 57% of AT–exposed patients. Among those with endoscopic data, mucosal healing was more frequent in AT–naïve (68%) vs. AT–exposed (41%). Dose escalation was independently associated with prior AT exposure (aOR 2.00, 95% CI 1.34–2.99) and UC/IBD-U subtype (aOR 1.81, 95% CI 1.26–2.61). In CD, AT exposure and higher weight were linked to escalation; in UC/IBD-U, higher weight was associated.
Discussion: Over half with IBD initiating ustekinumab required dose escalation, particularly those with prior AT exposure, UC, or higher weight. Despite this, ustekinumab was associated with high treatment persistence and favorable clinical and endoscopic outcomes.

Figure: Table 1. Baseline Characteristics of Patients with IBD initiating Ustekinumab.

Figure: Table 2. Outcomes of Patients Initiating Ustekinumab.
Disclosures:
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Sara Horst: Abbvie – Consultant. Biocon – Consultant. Celltrion – Consultant. J&J – Consultant. Pfizer – Consultant. Takeda – Consultant.
Audrey Bennett: Celltrion – Consultant. Pfizer – Grant/Research Support.
Anagha Ashokan indicated no relevant financial relationships.
Dania Hudhud indicated no relevant financial relationships.
Angela Wu indicated no relevant financial relationships.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Chelsea Anderson indicated no relevant financial relationships.
Vasantham Chaudhary indicated no relevant financial relationships.
Ellen Axenfeld indicated no relevant financial relationships.
Raymond K. Cross: Abbvie – Advisor or Review Panel Member, Consultant, Speakers Bureau. BMS – Advisor or Review Panel Member, Consultant, Speakers Bureau. CorEvitas – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member, Consultant. Genetech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. IBD Education Group – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Advisory Committee/Board Member, Consultant. Option Care – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member, Consultant. Samsung Bioepis – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Takeda – Grant/Research Support.
Jordan Axelrad, MD, MPH1, Sara Horst, MD, MPH, FACG2, Audrey Bennett, MD2, Anagha Ashokan, BS2, Dania Hudhud, MD3, Angela Wu, MD4, Millie D. Long, MD, FACG5, Chelsea Anderson, PhD, MPH6, Vasantham Chaudhary, MD7, Ellen M. Axenfeld, MD8, Raymond K. Cross, MD, MS, FACG9. P3332 - Predictors of Response to Ustekinumab and Identification of Factors Associated With Dose Escalation in Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
