Monday Poster Session
Category: IBD
P3319 - Early Response to Vedolizumab in Patients With Crohn's Disease in a Prospective Observational Study in Canada and US: The VOICE Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Millie D. Long, MD, FACG
Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Chapel Hill, NC
Presenting Author(s)
Vipul Jairath, MBChB, DPhil, MRCP1, Millie D. Long, MD, FACG2, Michael D. Kappelman, MD3, Gabriela Radulescu, MD4, Nicole Li, MSc4, Jingyang J. Wu, MSc5, Itzel Romo Bautista, MD6, Alessandra Insogna, PMP6, Christian Agboton, MD5, Neeraj Narula, MD7
1Department of Medicine and Department of Epidemiology and Biostatistics, Western University, London, ON, Canada; 2Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 3University of North Carolina, Chapel Hill, NC; 4Alimentiv Inc., London, ON, Canada; 5Takeda Pharmaceuticals, Cambridge, MA; 6Takeda Pharmaceuticals, Boston, MA; 7McMaster University, Hamilton, ON, Canada
Introduction: VOICE (NCT06249555) is a prospective, observational study in adult (≥18 yrs) pts with Crohn’s disease (CD) in Canada and the US initiating vedolizumab (VDZ) treatment (tx) as part of standard care. The aim is to explore the response to VDZ measured by Patient-Reported Outcomes Measurement Information System (PROMIS) Short Form (SF) scores in the following domains: pain interference, fatigue, anxiety, depression, physical function, sleep disturbance, and ability to participate in social roles or activities.
Methods: Inclusion criteria include baseline PROMIS Pain Interference (PI)-SF T-score ≥55 (0.5 SD above general population). The primary endpoint is time to first meaningful clinical improvement in PROMIS PI-SF T-score (≥2-point decrease from baseline). Other endpoints include: improvement in 7 PROMIS domains (5-point (0.5 SD) change in T-score from baseline); Short Inflammatory Bowel Disease Questionnaire (SIBDQ) improvement (≥9 point increase from baseline) and remission (score of ≥60); and safety. Here, we report results from pts with ≥1 VDZ dose and ≥1 post-baseline PI assessment included in an interim analysis (IA; prespecified to occur after 50 pts completed or discontinued before wk 14).
Results: Overall, 64 pts with ≥1 VDZ dose and ≥1 post-baseline PROMIS PI assessment (mean±SD age, 43.1±17.0 years, 35.9% male, 92.2% White) were included in the IA. Disease duration (mean±SD) was 6.4±12.1 years; 84.4% of pts were anti-TNF-naïve. Baseline PROMIS domain scores indicated notable impairment (Table). By wk 14, improvements from baseline were observed in all 7 measured domains, with ≥5-point improvements in 4/7 domains (Table). The median time to achieve the first ≥2-point decrease in PROMIS PI-SF T-score (n=56/64 pts) was 12 days (95% CI, 6-13). At wk 14, 37.5% (24/64) and 7.8% (5/64) pts achieved SIBDQ improvement and remission, respectively. One pt had serious adverse events of appendicitis and diverticulitis, and another pt had a fatal event; none were related to VDZ tx.
Discussion: This IA shows an early improvement across multiple symptom domains and in pts with CD starting VDZ tx. Time to first ≥2-point decrease in PROMIS PI-SF T-score was short, with a benefit observed after 12 days from VDZ initiation. Study recruitment with a planned sample size of 200 VDZ patients and a comparator group of 100 anti-IL-23 pts) and data collection are ongoing.
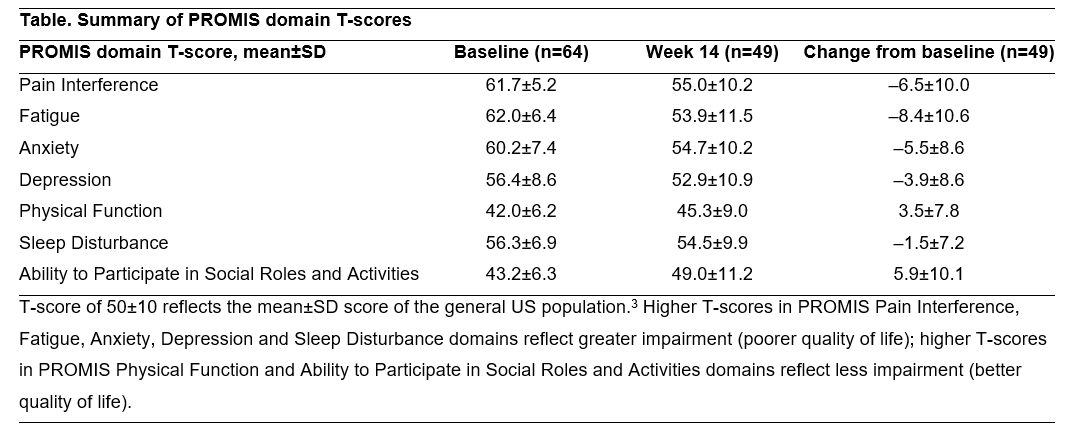
Figure: Table
Disclosures:
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Consultant. Enthera – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Consultant. JAMP – Consultant. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Syndegen – Consultant. Takeda – Consultant, Intellectual Property/Patents, Speakers Bureau. TD Securities – Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Michael Kappelman: Eli Lilly – Consultant. Takeda – Consultant.
Gabriela Radulescu: Alimentiv – Employee.
Nicole Li: Alimentiv – Employee.
Jingyang Wu: Takeda – Employee, Stock Options.
Itzel Romo Bautista: Takeda – Employee, Stock Options.
Alessandra Insogna: Takeda – Employee, Stock Options.
Christian Agboton: Takeda – Employee, Stock Options.
Neeraj Narula: Abbvie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Fresinius Kabi – Advisory Committee/Board Member, Consultant. Innomar Strategies – Advisory Committee/Board Member, Consultant. Iterative Health – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant.
Vipul Jairath, MBChB, DPhil, MRCP1, Millie D. Long, MD, FACG2, Michael D. Kappelman, MD3, Gabriela Radulescu, MD4, Nicole Li, MSc4, Jingyang J. Wu, MSc5, Itzel Romo Bautista, MD6, Alessandra Insogna, PMP6, Christian Agboton, MD5, Neeraj Narula, MD7. P3319 - Early Response to Vedolizumab in Patients With Crohn's Disease in a Prospective Observational Study in Canada and US: The VOICE Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Medicine and Department of Epidemiology and Biostatistics, Western University, London, ON, Canada; 2Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 3University of North Carolina, Chapel Hill, NC; 4Alimentiv Inc., London, ON, Canada; 5Takeda Pharmaceuticals, Cambridge, MA; 6Takeda Pharmaceuticals, Boston, MA; 7McMaster University, Hamilton, ON, Canada
Introduction: VOICE (NCT06249555) is a prospective, observational study in adult (≥18 yrs) pts with Crohn’s disease (CD) in Canada and the US initiating vedolizumab (VDZ) treatment (tx) as part of standard care. The aim is to explore the response to VDZ measured by Patient-Reported Outcomes Measurement Information System (PROMIS) Short Form (SF) scores in the following domains: pain interference, fatigue, anxiety, depression, physical function, sleep disturbance, and ability to participate in social roles or activities.
Methods: Inclusion criteria include baseline PROMIS Pain Interference (PI)-SF T-score ≥55 (0.5 SD above general population). The primary endpoint is time to first meaningful clinical improvement in PROMIS PI-SF T-score (≥2-point decrease from baseline). Other endpoints include: improvement in 7 PROMIS domains (5-point (0.5 SD) change in T-score from baseline); Short Inflammatory Bowel Disease Questionnaire (SIBDQ) improvement (≥9 point increase from baseline) and remission (score of ≥60); and safety. Here, we report results from pts with ≥1 VDZ dose and ≥1 post-baseline PI assessment included in an interim analysis (IA; prespecified to occur after 50 pts completed or discontinued before wk 14).
Results: Overall, 64 pts with ≥1 VDZ dose and ≥1 post-baseline PROMIS PI assessment (mean±SD age, 43.1±17.0 years, 35.9% male, 92.2% White) were included in the IA. Disease duration (mean±SD) was 6.4±12.1 years; 84.4% of pts were anti-TNF-naïve. Baseline PROMIS domain scores indicated notable impairment (Table). By wk 14, improvements from baseline were observed in all 7 measured domains, with ≥5-point improvements in 4/7 domains (Table). The median time to achieve the first ≥2-point decrease in PROMIS PI-SF T-score (n=56/64 pts) was 12 days (95% CI, 6-13). At wk 14, 37.5% (24/64) and 7.8% (5/64) pts achieved SIBDQ improvement and remission, respectively. One pt had serious adverse events of appendicitis and diverticulitis, and another pt had a fatal event; none were related to VDZ tx.
Discussion: This IA shows an early improvement across multiple symptom domains and in pts with CD starting VDZ tx. Time to first ≥2-point decrease in PROMIS PI-SF T-score was short, with a benefit observed after 12 days from VDZ initiation. Study recruitment with a planned sample size of 200 VDZ patients and a comparator group of 100 anti-IL-23 pts) and data collection are ongoing.

Figure: Table
Disclosures:
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Consultant. Enthera – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Consultant. JAMP – Consultant. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Syndegen – Consultant. Takeda – Consultant, Intellectual Property/Patents, Speakers Bureau. TD Securities – Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Michael Kappelman: Eli Lilly – Consultant. Takeda – Consultant.
Gabriela Radulescu: Alimentiv – Employee.
Nicole Li: Alimentiv – Employee.
Jingyang Wu: Takeda – Employee, Stock Options.
Itzel Romo Bautista: Takeda – Employee, Stock Options.
Alessandra Insogna: Takeda – Employee, Stock Options.
Christian Agboton: Takeda – Employee, Stock Options.
Neeraj Narula: Abbvie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Fresinius Kabi – Advisory Committee/Board Member, Consultant. Innomar Strategies – Advisory Committee/Board Member, Consultant. Iterative Health – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant.
Vipul Jairath, MBChB, DPhil, MRCP1, Millie D. Long, MD, FACG2, Michael D. Kappelman, MD3, Gabriela Radulescu, MD4, Nicole Li, MSc4, Jingyang J. Wu, MSc5, Itzel Romo Bautista, MD6, Alessandra Insogna, PMP6, Christian Agboton, MD5, Neeraj Narula, MD7. P3319 - Early Response to Vedolizumab in Patients With Crohn's Disease in a Prospective Observational Study in Canada and US: The VOICE Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
