Monday Poster Session
Category: IBD
P3315 - Mirikizumab Provides Sustained Long-Term Efficacy Up to 4 Years of Treatment for Ulcerative Colitis: Final Results From the LUCENT-3 Open-Label Extension Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Peter M. Irving, MD
IBD Unit, Guy’s and St Thomas’ Hospital, London, UK
London, England, United Kingdom
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, David B.. Clemow, PhD2, Geert R. D’Haens, MD, PhD3, Severine Vermeire, MD, PhD4, Peter M. Irving, MD5, Taku Kobayashi, 6, Laurent Peyrin-Biroulet, MD, PhD7, Karen Samaan, 2, Anil Gaur, 2, Jerome Paulissen, 2, Sarah Folian, 2, Ravneet Arora, 2, Nicholas Paquette, 8, Richard E.. Moses, DO, JD2, Axel Dignass, MD, PhD9
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Eli Lilly and Company, Indianapolis, IN; 3Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Department of Gastroenterology & Hepatology, University hospital Leuven, Leuven, Brabant Wallon, Belgium; 5IBD Unit, Guy’s and St Thomas’ Hospital, London, UK, London, England, United Kingdom; 6Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Tokyo, Japan; 7Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 8Syneos Health, Morrisville, NC; 9Agaplesion Markus Hospital, Frankfurt, Hessen, Germany
Introduction: Mirikizumab, a p19-directed interleukin-23 monoclonal antibody, is efficacious in inducing clinical remission at Week (W) 12 and maintaining clinical remission through W152 in moderately-to-severely active ulcerative colitis. Here, we present efficacy and safety results through W212 of mirikizumab treatment from the open-label extension LUCENT-3 study.
Methods: Symptomatic, clinical, endoscopic, histologic, corticosteroid-free (CSF), bowel urgency, quality-of-life, and adverse event (AE) outcomes are reported for mirikizumab induction responders, including patients with biologic failure, who entered LUCENT-3, with data shown for W52 maintenance remitters. Discontinuations or missing data were handled by nonresponder imputation (NRI), modified NRI (mNRI), and observed case (OC). mNRI uses multiple imputation for missing data and balances bias of NRI and OC. Table 1 provides endpoint and population definitions and abbreviations for maintenance remitters. Safety population N=339.
Results: Using mNRI (N=179), among W52 mirikizumab maintenance remitters demonstrated clinical response in 79% at W212. W212 remission rates among W52 clinical remitters were 77% for symptomatic, 62% for clinical, 66% for endoscopic, 53% for histologic-endoscopic mucosal, 62% for CSF, and 60% for bowel urgency; 78% for Inflammatory Bowel Disease Questionnaire (IBDQ) response, and 73% IBDQ remission. Histologic-endoscopic mucosal improvement and bowel urgency clinically meaningful improvement at W212 were achieved in 54% and 75% of patients, respectively. Biologic Failed/Not Biologic Failed subgroup data demonstrated maintenance of efficacy (Table 1). Stool frequency, rectal bleeding, bowel urgency, and abdominal pain symptom score reductions from induction baseline at W52 were sustained through W212. For W0-160 of LUCENT-3, serious AEs were reported in 12% patients, while 7% discontinued treatment due to an AE. AEs of special interest: opportunistic infection (4.4%), cerebrocardiovascular events (1.5%), and malignancy (1.5%). Liver enzymes ≥3 upper limit of normal (ULN) included alanine aminotransferase (2%) and aspartate aminotransferase (1%); total bilirubin level was ≥2 ULN (2%).
Discussion: Mirikizumab provides long-term symptomatic, clinical, endoscopic, histologic, corticosteroid-free, and quality-of-life sustained and durable maintenance of benefits up to 4 years in patients with UC, including for biologic failed patients, with no new safety concerns.
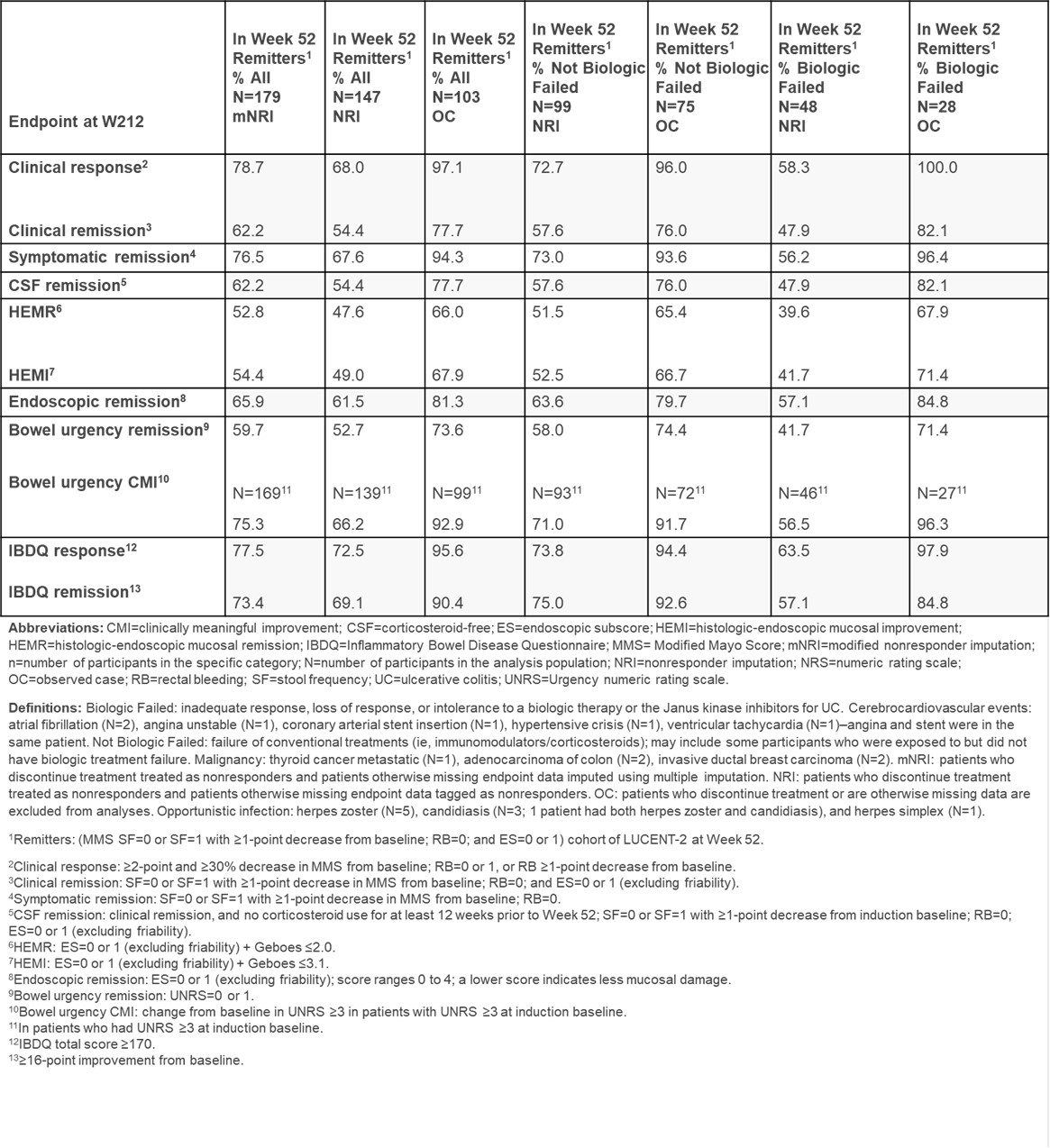
Figure: Summary of Mirikizumab Efficacy at Week 212 in Week 52 Remitters.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
David Clemow: Eli Lilly and Company – Employee, Stock Options.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Severine Vermeire: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AbolerIS Pharma – Consultant, Speakers Bureau. AgomAb – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Avaxia – Consultant, Speakers Bureau. BMS – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech-Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIdomics – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. Mestag Therapeutics, – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prodigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillots Pharma AG – Consultant, Speakers Bureau. VectivBio – Consultant, Speakers Bureau. Ventyx – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Peter Irving: Arena – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisor or Review Panel Member. Gilead – Advisor or Review Panel Member, Speakers Bureau. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. MSD – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Pharmacosmos – Advisor or Review Panel Member. Prometheus – Advisor or Review Panel Member. Roche – Advisor or Review Panel Member. Samsung Bioepis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. Topivert – Advisor or Review Panel Member. VH2 – Advisor or Review Panel Member. Vifor Pharma – Advisor or Review Panel Member. Warner Chilcott – Advisor or Review Panel Member, Speakers Bureau.
Taku Kobayashi: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ajinomoto Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Alfresa Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Medical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Astellas – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Covidien – Advisory Committee/Board Member, Consultant, Speakers Bureau. EA Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Eisai – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. JIMRO – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kyorin Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mitsubishi Tanabe Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mochida Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Nippon Kayaku – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Otsuka Holdings – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sekisui Medical – Grant/Research Support. Takeda Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Thermo Scientific – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Zeria Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Karen Samaan: Eli Lilly and Company – Employee, Stock Options.
Anil Gaur: Eli Lilly and Company – Employee, Stock Options.
Jerome Paulissen: Eli Lilly and Company – Employee, Stock Options.
Sarah Folian: Eli Lilly and Company – Employee, Stock Options.
Ravneet Arora: Eli Lilly and Company – Employee, Stock Options.
Nicholas Paquette: Syneos Health – Employee.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Axel Dignass: Abbvie – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Abivax – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Johnson & Johnson – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Lilly – Consultant. Materia – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Prima – Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Stada – Consultant. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tilliots – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Bruce E. Sands, MD, MS, FACG1, David B.. Clemow, PhD2, Geert R. D’Haens, MD, PhD3, Severine Vermeire, MD, PhD4, Peter M. Irving, MD5, Taku Kobayashi, 6, Laurent Peyrin-Biroulet, MD, PhD7, Karen Samaan, 2, Anil Gaur, 2, Jerome Paulissen, 2, Sarah Folian, 2, Ravneet Arora, 2, Nicholas Paquette, 8, Richard E.. Moses, DO, JD2, Axel Dignass, MD, PhD9. P3315 - Mirikizumab Provides Sustained Long-Term Efficacy Up to 4 Years of Treatment for Ulcerative Colitis: Final Results From the LUCENT-3 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Eli Lilly and Company, Indianapolis, IN; 3Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Department of Gastroenterology & Hepatology, University hospital Leuven, Leuven, Brabant Wallon, Belgium; 5IBD Unit, Guy’s and St Thomas’ Hospital, London, UK, London, England, United Kingdom; 6Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Tokyo, Japan; 7Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 8Syneos Health, Morrisville, NC; 9Agaplesion Markus Hospital, Frankfurt, Hessen, Germany
Introduction: Mirikizumab, a p19-directed interleukin-23 monoclonal antibody, is efficacious in inducing clinical remission at Week (W) 12 and maintaining clinical remission through W152 in moderately-to-severely active ulcerative colitis. Here, we present efficacy and safety results through W212 of mirikizumab treatment from the open-label extension LUCENT-3 study.
Methods: Symptomatic, clinical, endoscopic, histologic, corticosteroid-free (CSF), bowel urgency, quality-of-life, and adverse event (AE) outcomes are reported for mirikizumab induction responders, including patients with biologic failure, who entered LUCENT-3, with data shown for W52 maintenance remitters. Discontinuations or missing data were handled by nonresponder imputation (NRI), modified NRI (mNRI), and observed case (OC). mNRI uses multiple imputation for missing data and balances bias of NRI and OC. Table 1 provides endpoint and population definitions and abbreviations for maintenance remitters. Safety population N=339.
Results: Using mNRI (N=179), among W52 mirikizumab maintenance remitters demonstrated clinical response in 79% at W212. W212 remission rates among W52 clinical remitters were 77% for symptomatic, 62% for clinical, 66% for endoscopic, 53% for histologic-endoscopic mucosal, 62% for CSF, and 60% for bowel urgency; 78% for Inflammatory Bowel Disease Questionnaire (IBDQ) response, and 73% IBDQ remission. Histologic-endoscopic mucosal improvement and bowel urgency clinically meaningful improvement at W212 were achieved in 54% and 75% of patients, respectively. Biologic Failed/Not Biologic Failed subgroup data demonstrated maintenance of efficacy (Table 1). Stool frequency, rectal bleeding, bowel urgency, and abdominal pain symptom score reductions from induction baseline at W52 were sustained through W212. For W0-160 of LUCENT-3, serious AEs were reported in 12% patients, while 7% discontinued treatment due to an AE. AEs of special interest: opportunistic infection (4.4%), cerebrocardiovascular events (1.5%), and malignancy (1.5%). Liver enzymes ≥3 upper limit of normal (ULN) included alanine aminotransferase (2%) and aspartate aminotransferase (1%); total bilirubin level was ≥2 ULN (2%).
Discussion: Mirikizumab provides long-term symptomatic, clinical, endoscopic, histologic, corticosteroid-free, and quality-of-life sustained and durable maintenance of benefits up to 4 years in patients with UC, including for biologic failed patients, with no new safety concerns.

Figure: Summary of Mirikizumab Efficacy at Week 212 in Week 52 Remitters.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
David Clemow: Eli Lilly and Company – Employee, Stock Options.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Severine Vermeire: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AbolerIS Pharma – Consultant, Speakers Bureau. AgomAb – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Avaxia – Consultant, Speakers Bureau. BMS – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech-Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIdomics – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. Mestag Therapeutics, – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prodigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillots Pharma AG – Consultant, Speakers Bureau. VectivBio – Consultant, Speakers Bureau. Ventyx – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Peter Irving: Arena – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisor or Review Panel Member. Gilead – Advisor or Review Panel Member, Speakers Bureau. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. MSD – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Pharmacosmos – Advisor or Review Panel Member. Prometheus – Advisor or Review Panel Member. Roche – Advisor or Review Panel Member. Samsung Bioepis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. Topivert – Advisor or Review Panel Member. VH2 – Advisor or Review Panel Member. Vifor Pharma – Advisor or Review Panel Member. Warner Chilcott – Advisor or Review Panel Member, Speakers Bureau.
Taku Kobayashi: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ajinomoto Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Alfresa Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Medical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Astellas – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Covidien – Advisory Committee/Board Member, Consultant, Speakers Bureau. EA Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Eisai – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. JIMRO – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kyorin Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mitsubishi Tanabe Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mochida Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Nippon Kayaku – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Otsuka Holdings – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sekisui Medical – Grant/Research Support. Takeda Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Thermo Scientific – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Zeria Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Karen Samaan: Eli Lilly and Company – Employee, Stock Options.
Anil Gaur: Eli Lilly and Company – Employee, Stock Options.
Jerome Paulissen: Eli Lilly and Company – Employee, Stock Options.
Sarah Folian: Eli Lilly and Company – Employee, Stock Options.
Ravneet Arora: Eli Lilly and Company – Employee, Stock Options.
Nicholas Paquette: Syneos Health – Employee.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Axel Dignass: Abbvie – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Abivax – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Johnson & Johnson – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Lilly – Consultant. Materia – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Prima – Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Stada – Consultant. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tilliots – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Bruce E. Sands, MD, MS, FACG1, David B.. Clemow, PhD2, Geert R. D’Haens, MD, PhD3, Severine Vermeire, MD, PhD4, Peter M. Irving, MD5, Taku Kobayashi, 6, Laurent Peyrin-Biroulet, MD, PhD7, Karen Samaan, 2, Anil Gaur, 2, Jerome Paulissen, 2, Sarah Folian, 2, Ravneet Arora, 2, Nicholas Paquette, 8, Richard E.. Moses, DO, JD2, Axel Dignass, MD, PhD9. P3315 - Mirikizumab Provides Sustained Long-Term Efficacy Up to 4 Years of Treatment for Ulcerative Colitis: Final Results From the LUCENT-3 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
