Monday Poster Session
Category: IBD
P3272 - Early Response of Mirikizumab Therapy for Ulcerative Colitis Patients in a Real-World Setting
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Hannah O'Neill, MD (she/her/hers)
Houston Methodist-Weill Cornell Graduate School of Medical Sciences
Houston, TX
Presenting Author(s)
Hannah O'Neill, MD1, Orhun Davarci, MS2, Christopher Fan, MD3, Bincy Abraham, MD, MS, FACG4
1Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX; 2Texas A&M School of Engineering Medicine, Houston Methodist Hospital, Houston, TX; 3Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine / Weill Cornell Medical College, Cornell University, Houston, TX; 4Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX
Introduction: Ulcerative colitis (UC) is a debilitating condition with high morbidity and mortality1. Despite many available advanced therapies, there is a patient subset with persistent, moderate-severely active UC.
Mirikizumab (MIRI) is an IL23 inhibitor recently approved for UC patients with moderate-severe disease2. The LUCENT-1 induction trial assessed MIRI efficacy for patients with 0 to 2 therapy failures3,4,5. This study looks at MIRI induction in a real-world cohort.
Methods: This retrospective, single-center study included UC patients who received MIRI (2023 – 2024). Baseline characteristics were assessed to determine response predictors. Variables included demographics, disease severity, blood and stool biomarkers, location (Montreal classification), and historic therapies. Clinical parameters were Mayo score and Simple Clinical Colitis Activity Index (SSCAI). Exclusion criteria included lack of MIRI initiation / follow-up and LUCENT inclusion.
Results: Of 25 initial patients prescribed MIRI, 16 were analyzed; exclusion was due to lack of initiation (5) / follow-up (3) or LUCENT inclusion (1). Fifty percent were female, 88% Caucasian, and 13% Hispanic. Twelve patients (75%) had pancolitis and an average of 3 prior advanced therapies. Fifteen (94%) failed a TNF-α inhibitor, and 44% failed IL12/IL23 (ustekinumab). Twelve patients (75%) had symptomatic improvement (partial Mayo) during induction. They had higher baseline partial Mayo / SSCAI, a greater reduction in the same, and more reduction of fecal calprotectin. These 12 were on therapy longer (3 vs 1.5 months). Intestinal ultrasound (IUS) had complete transmural healing (2 patients). Interval colonoscopy at 2 months (2 patients) did not yet reveal endoscopic improvement, however, both had symptomatic improvement. One patient discontinued MIRI – she had persistent inflammation per IUS and colonoscopy 6 months into therapy.
Discussion: This retrospective study of MIRI induction noted symptomatic improvement based on clinical scores and biomarker improvement. The patient subset without improvement was on therapy for a shorter period of time, likely contributing to limited response. In the patient subgroup who underwent further evaluation, IUS demonstrated transmural healing, while colonoscopy had not yet demonstrated improvement. We suspect short time on therapy limited endoscopic evaluation. All but one patient continued MIRI post-induction. In this study, MIRI improved symptoms and inflammatory markers in the majority of patients.
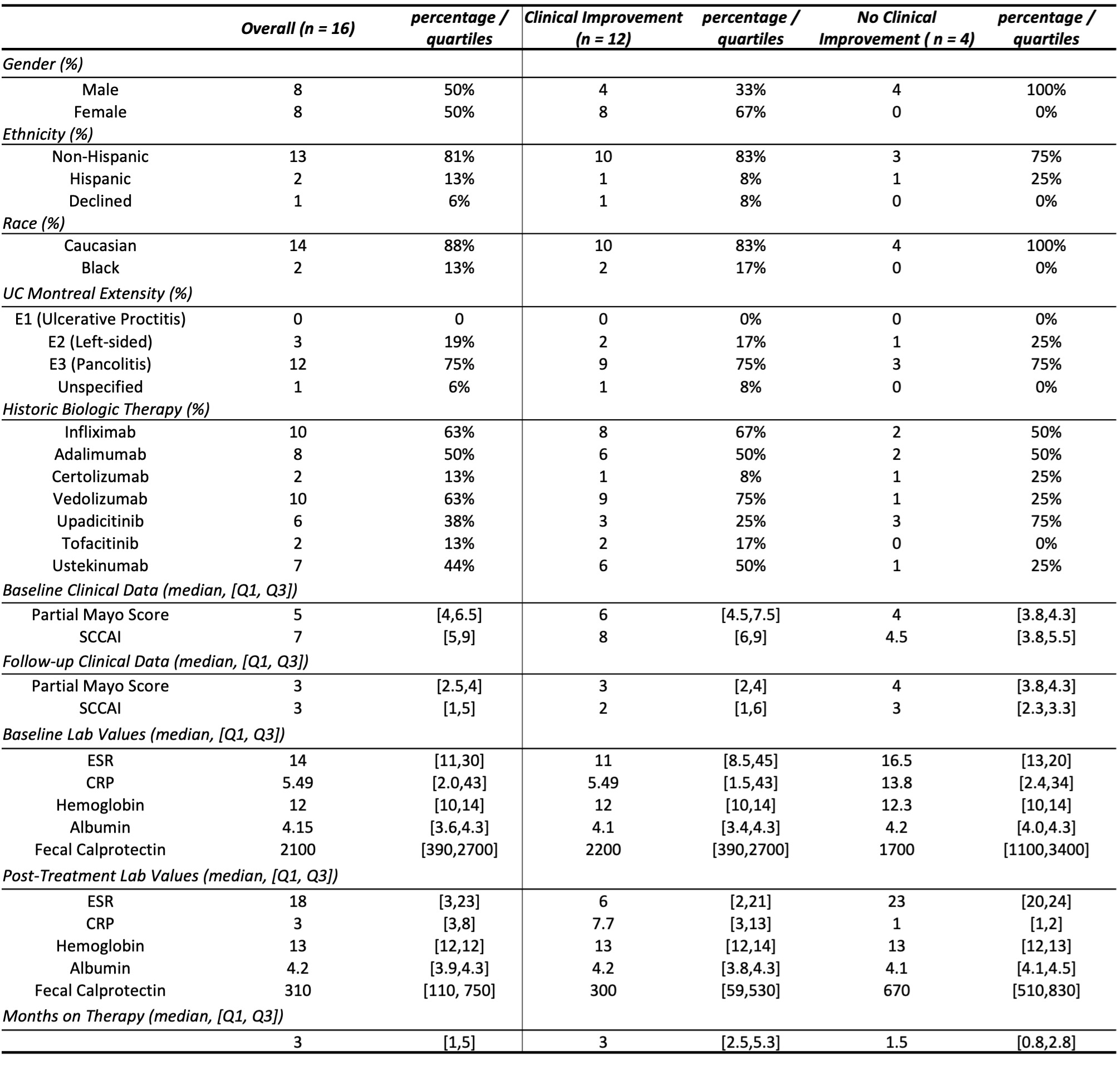
Figure: Table 1: Comparison of Patients on Mirikizumab Induction Therapy With and Without Clinical Improvement Over the Induction Period
Abbreviations: Ulcerative Colitis (UC), Simple Clinical Colitis Activity Index (SCCAI), Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP)
Disclosures:
Hannah O'Neill indicated no relevant financial relationships.
Orhun Davarci indicated no relevant financial relationships.
Christopher Fan indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Hannah O'Neill, MD1, Orhun Davarci, MS2, Christopher Fan, MD3, Bincy Abraham, MD, MS, FACG4. P3272 - Early Response of Mirikizumab Therapy for Ulcerative Colitis Patients in a Real-World Setting, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX; 2Texas A&M School of Engineering Medicine, Houston Methodist Hospital, Houston, TX; 3Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine / Weill Cornell Medical College, Cornell University, Houston, TX; 4Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX
Introduction: Ulcerative colitis (UC) is a debilitating condition with high morbidity and mortality1. Despite many available advanced therapies, there is a patient subset with persistent, moderate-severely active UC.
Mirikizumab (MIRI) is an IL23 inhibitor recently approved for UC patients with moderate-severe disease2. The LUCENT-1 induction trial assessed MIRI efficacy for patients with 0 to 2 therapy failures3,4,5. This study looks at MIRI induction in a real-world cohort.
Methods: This retrospective, single-center study included UC patients who received MIRI (2023 – 2024). Baseline characteristics were assessed to determine response predictors. Variables included demographics, disease severity, blood and stool biomarkers, location (Montreal classification), and historic therapies. Clinical parameters were Mayo score and Simple Clinical Colitis Activity Index (SSCAI). Exclusion criteria included lack of MIRI initiation / follow-up and LUCENT inclusion.
Results: Of 25 initial patients prescribed MIRI, 16 were analyzed; exclusion was due to lack of initiation (5) / follow-up (3) or LUCENT inclusion (1). Fifty percent were female, 88% Caucasian, and 13% Hispanic. Twelve patients (75%) had pancolitis and an average of 3 prior advanced therapies. Fifteen (94%) failed a TNF-α inhibitor, and 44% failed IL12/IL23 (ustekinumab). Twelve patients (75%) had symptomatic improvement (partial Mayo) during induction. They had higher baseline partial Mayo / SSCAI, a greater reduction in the same, and more reduction of fecal calprotectin. These 12 were on therapy longer (3 vs 1.5 months). Intestinal ultrasound (IUS) had complete transmural healing (2 patients). Interval colonoscopy at 2 months (2 patients) did not yet reveal endoscopic improvement, however, both had symptomatic improvement. One patient discontinued MIRI – she had persistent inflammation per IUS and colonoscopy 6 months into therapy.
Discussion: This retrospective study of MIRI induction noted symptomatic improvement based on clinical scores and biomarker improvement. The patient subset without improvement was on therapy for a shorter period of time, likely contributing to limited response. In the patient subgroup who underwent further evaluation, IUS demonstrated transmural healing, while colonoscopy had not yet demonstrated improvement. We suspect short time on therapy limited endoscopic evaluation. All but one patient continued MIRI post-induction. In this study, MIRI improved symptoms and inflammatory markers in the majority of patients.

Figure: Table 1: Comparison of Patients on Mirikizumab Induction Therapy With and Without Clinical Improvement Over the Induction Period
Abbreviations: Ulcerative Colitis (UC), Simple Clinical Colitis Activity Index (SCCAI), Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP)
Disclosures:
Hannah O'Neill indicated no relevant financial relationships.
Orhun Davarci indicated no relevant financial relationships.
Christopher Fan indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Hannah O'Neill, MD1, Orhun Davarci, MS2, Christopher Fan, MD3, Bincy Abraham, MD, MS, FACG4. P3272 - Early Response of Mirikizumab Therapy for Ulcerative Colitis Patients in a Real-World Setting, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
