Monday Poster Session
Category: IBD
P3260 - Fecal Microbiota Transplantation Associated With Clinical and Endoscopic Remission in Ulcerative Colitis: A Meta-Analysis of Randomized Control Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- RT
Rahul Tripathi, MD
Stony Brook Medicine
Stony Brook, NY
Presenting Author(s)
Rahul Tripathi, MD1, James Lee, MD2, Jordan Barnett-Kradjian, DO1, Matthew Pelletier, DO1, Daniel Jamorabo, MD3
1Stony Brook Medicine, Stony Brook, NY; 2Stony Brook University Hospital, Stony Brook, NY; 3Northwell Health, Forest Hills, NY
Introduction: Despite advances in medical therapy, a significant proportion of patients with ulcerative colitis (UC) fail to achieve sustained remission. Fecal microbiota transplantation (FMT), which involves transferring stool from a healthy donor to a patient’s gastrointestinal tract, has emerged as a promising therapy for restoring gut microbial balance. While early randomized controlled trials (RCTs) suggested benefit, more recent studies have yielded mixed results. We conducted a meta-analysis of RCTs comparing FMT to placebo or standard therapy in UC patients to evaluate its efficacy in inducing clinical remission.
Methods: A systematic search of PubMed, Embase, and Web of Science was performed from inception through May 2025 to identify RCTs evaluating FMT versus placebo or standard therapy in UC. Eligible studies included those reporting clinical remission rates and using any method of FMT delivery. The primary outcome was clinical remission, as defined by each study. Secondary outcomes included endoscopic improvement and gastrointestinal (GI) adverse effects. Event rates and total participants were extracted, and pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using a random-effects model via the Mantel-Haenszel method.
Results: Seventeen RCTs comprising 802 patients (400 FMT, 402 control) were included. FMT was associated with significantly higher rates of clinical remission compared to control (RR 1.53; 95% CI: 1.17–2.00; P = 0.0019). Among six studies reporting endoscopic improvement (173 FMT vs. 162 control), FMT conferred a significant benefit (RR 1.72; 95% CI: 1.23–2.39; P = 0.0013). GI adverse effects, reported in ten studies, were similar between groups (RR 0.92; 95% CI: 0.82–1.04; P = 0.17), supporting a favorable safety profile.
Discussion: This meta-analysis demonstrates that FMT significantly improves clinical remission and endoscopic outcomes in UC patients compared to placebo or standard therapy, without increasing gastrointestinal adverse events. These findings support FMT as a safe and effective therapeutic option, particularly for patients who are refractory to conventional treatments. Future research should focus on optimizing delivery methods, donor selection, and strategies for sustained response.
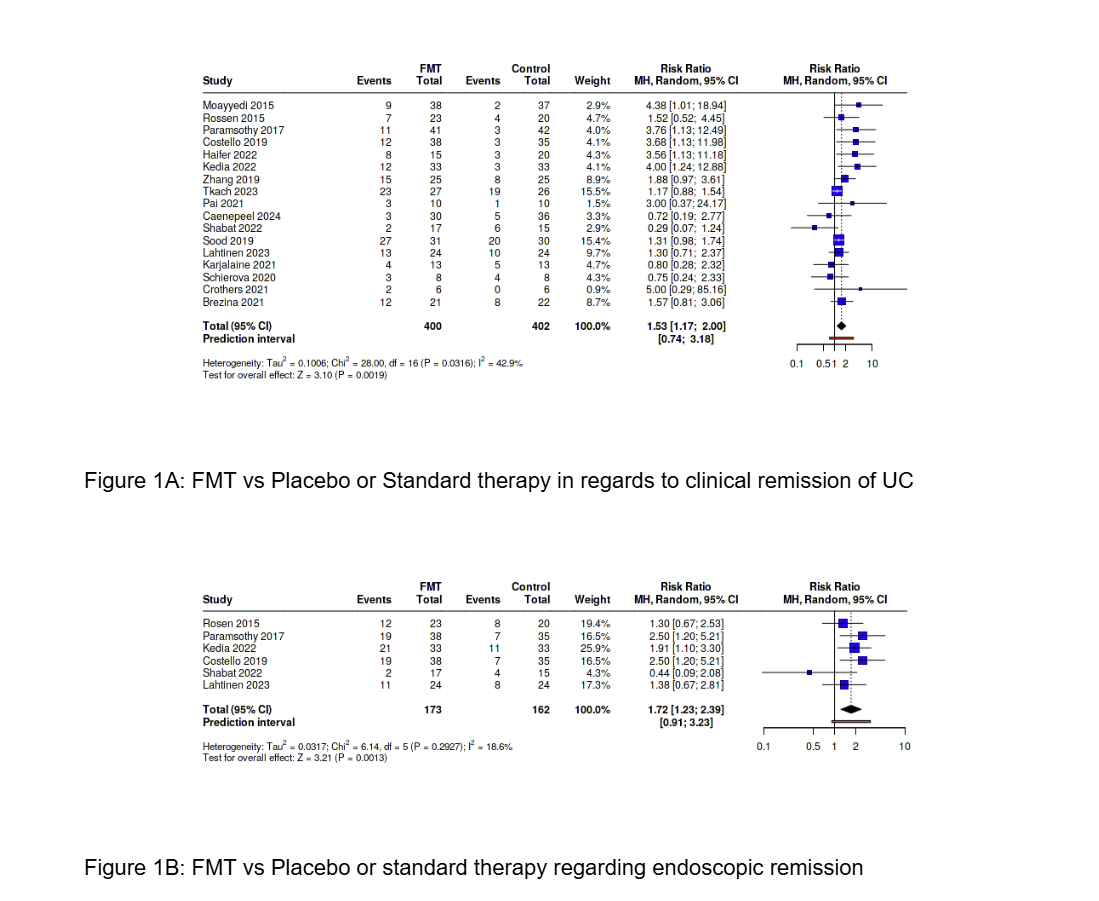
Figure: Figure 1A: FMT vs Placebo or Standard therapy in regards to clinical remission of UC
Figure 1B: FMT vs Placebo or standard therapy regarding endoscopic remission
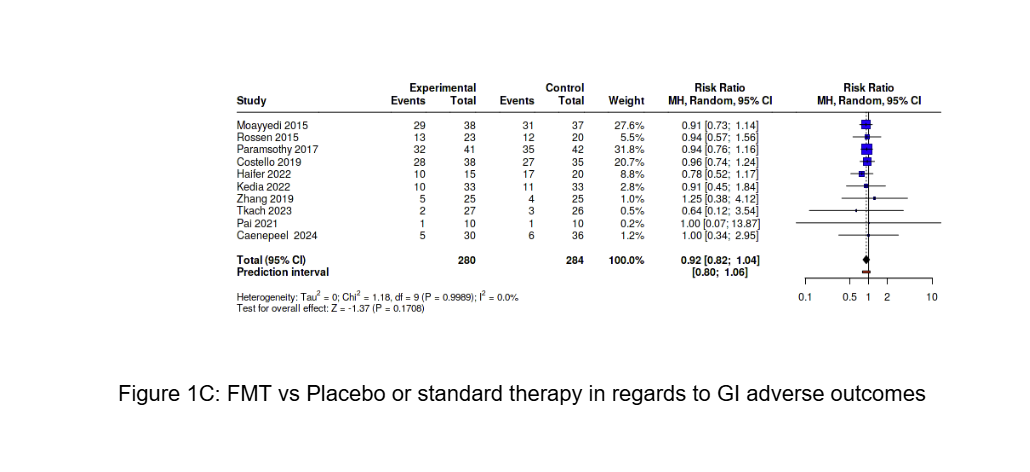
Figure: Figure 1C: FMT vs Placebo or standard therapy in regards to GI adverse outcomes
Disclosures:
Rahul Tripathi indicated no relevant financial relationships.
James Lee indicated no relevant financial relationships.
Jordan Barnett-Kradjian indicated no relevant financial relationships.
Matthew Pelletier indicated no relevant financial relationships.
Daniel Jamorabo indicated no relevant financial relationships.
Rahul Tripathi, MD1, James Lee, MD2, Jordan Barnett-Kradjian, DO1, Matthew Pelletier, DO1, Daniel Jamorabo, MD3. P3260 - Fecal Microbiota Transplantation Associated With Clinical and Endoscopic Remission in Ulcerative Colitis: A Meta-Analysis of Randomized Control Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Stony Brook Medicine, Stony Brook, NY; 2Stony Brook University Hospital, Stony Brook, NY; 3Northwell Health, Forest Hills, NY
Introduction: Despite advances in medical therapy, a significant proportion of patients with ulcerative colitis (UC) fail to achieve sustained remission. Fecal microbiota transplantation (FMT), which involves transferring stool from a healthy donor to a patient’s gastrointestinal tract, has emerged as a promising therapy for restoring gut microbial balance. While early randomized controlled trials (RCTs) suggested benefit, more recent studies have yielded mixed results. We conducted a meta-analysis of RCTs comparing FMT to placebo or standard therapy in UC patients to evaluate its efficacy in inducing clinical remission.
Methods: A systematic search of PubMed, Embase, and Web of Science was performed from inception through May 2025 to identify RCTs evaluating FMT versus placebo or standard therapy in UC. Eligible studies included those reporting clinical remission rates and using any method of FMT delivery. The primary outcome was clinical remission, as defined by each study. Secondary outcomes included endoscopic improvement and gastrointestinal (GI) adverse effects. Event rates and total participants were extracted, and pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using a random-effects model via the Mantel-Haenszel method.
Results: Seventeen RCTs comprising 802 patients (400 FMT, 402 control) were included. FMT was associated with significantly higher rates of clinical remission compared to control (RR 1.53; 95% CI: 1.17–2.00; P = 0.0019). Among six studies reporting endoscopic improvement (173 FMT vs. 162 control), FMT conferred a significant benefit (RR 1.72; 95% CI: 1.23–2.39; P = 0.0013). GI adverse effects, reported in ten studies, were similar between groups (RR 0.92; 95% CI: 0.82–1.04; P = 0.17), supporting a favorable safety profile.
Discussion: This meta-analysis demonstrates that FMT significantly improves clinical remission and endoscopic outcomes in UC patients compared to placebo or standard therapy, without increasing gastrointestinal adverse events. These findings support FMT as a safe and effective therapeutic option, particularly for patients who are refractory to conventional treatments. Future research should focus on optimizing delivery methods, donor selection, and strategies for sustained response.

Figure: Figure 1A: FMT vs Placebo or Standard therapy in regards to clinical remission of UC
Figure 1B: FMT vs Placebo or standard therapy regarding endoscopic remission

Figure: Figure 1C: FMT vs Placebo or standard therapy in regards to GI adverse outcomes
Disclosures:
Rahul Tripathi indicated no relevant financial relationships.
James Lee indicated no relevant financial relationships.
Jordan Barnett-Kradjian indicated no relevant financial relationships.
Matthew Pelletier indicated no relevant financial relationships.
Daniel Jamorabo indicated no relevant financial relationships.
Rahul Tripathi, MD1, James Lee, MD2, Jordan Barnett-Kradjian, DO1, Matthew Pelletier, DO1, Daniel Jamorabo, MD3. P3260 - Fecal Microbiota Transplantation Associated With Clinical and Endoscopic Remission in Ulcerative Colitis: A Meta-Analysis of Randomized Control Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
