Monday Poster Session
Category: IBD
P3245 - Longer Sustained Remission in Crohn’s Disease With Guselkumab versus Ustekinumab Treatment: Projections From a Disease Model
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Myrlene Sanon, MPH
Johnson & Johnson
Metuchen, NJ
Presenting Author(s)
Mariya Dimova, 1, Mi Jun Keng, PhD2, Feng Pan, PhD3, Dominik Naessens, PharmD, MBA4, Leonardo Salese, MD5, Zijiang Yang, PhD5, Sumesh Kachroo, PhD6, Mario A. Gomez Camacho, MD, PhD, MSc, MBA7, Myrlene Sanon, MPH8
1Janssen-Cilag, Issy-les-Moulineaux, Centre, France; 2Johnson & Johnson, Beerse, Antwerpen, Belgium; 3Johnson & Johnson, Raritan, NJ; 4Janssen Pharmaceutica N.V., Beerse, Brussels Hoofdstedelijk Gewest, Belgium; 5Johnson & Johnson, Spring House, PA; 6Johnson & Johnson, Horsham, PA; 7Johnson & Johnson, Philadelphia, PA; 8Johnson & Johnson, Metuchen, NJ
Introduction: Crohn’s disease (CD) is a progressive disease associated with reduced quality of life. Therapeutic goals and clinical guidelines have evolved to include endoscopic outcomes as a key prognostic parameter in disease management (STRIDE II). Guselkumab, a dual-acting IL-23 inhibitor, investigated in double-blind placebo-control, active comparator, Phase 3 trials, demonstrated superiority in 48-week efficacy to ustekinumab in achieving endoscopic response with 52.7% (p< 0.001) for guselkumab 200mg, and 47.9% (p=0.009) for guselkumab 100mg vs 37.1% for ustekinumab. To better understand the implication of endoscopic response on long-term remission, a disease model was developed to compare long-term disease clinical remission status for guselkumab and ustekinumab.
Methods: A state-transition model was developed to simulate long-term disease status change for patients with CD who initiate guselkumab or ustekinumab. In the model, patients can be in one of the three health states – clinical remission, clinical response without clinical remission, and active CD. For the first 48 weeks, the rates of clinical remission and clinical response are based on the GALAXI Phase 3 trials. After week 48, endoscopic response status based on galaxi 2-year long-term extension data were used to estimate the durability of clinical remission defined as those achieving clinical remission at each year over a 5-year period. Overtime, patients losing clinical remission or response will move to the active CD state.
Results: The model predicts the proportion of patients in clinical remission for guselkumab compared to ustekinumab at year two was 56.6% versus 49.7%, year three was 47.2% versus 37.9%, and year five was 32.9% versus 22.7% (Figure). The difference in proportion of patients attaining clinical remission with guselkumab compared to ustekinumab increases from 5% at year 1 (68% vs. 63%) to 10% at year 5 (33% vs. 23%). Model predicts over 5 years, among patients who achieved clinical remission at 1 year, 49% maintained clinical remission on guselkumab versus 36% on ustekinumab (Table).
Discussion: The model projections suggest treatment with guselkumab leads to better long-term outcomes for patients with CD, compared to ustekinumab. The results support the utility of endoscopic response as a predictor of long-term outcomes and suggests longer sustained clinical remission with guselkumab treatment versus ustekinumab.
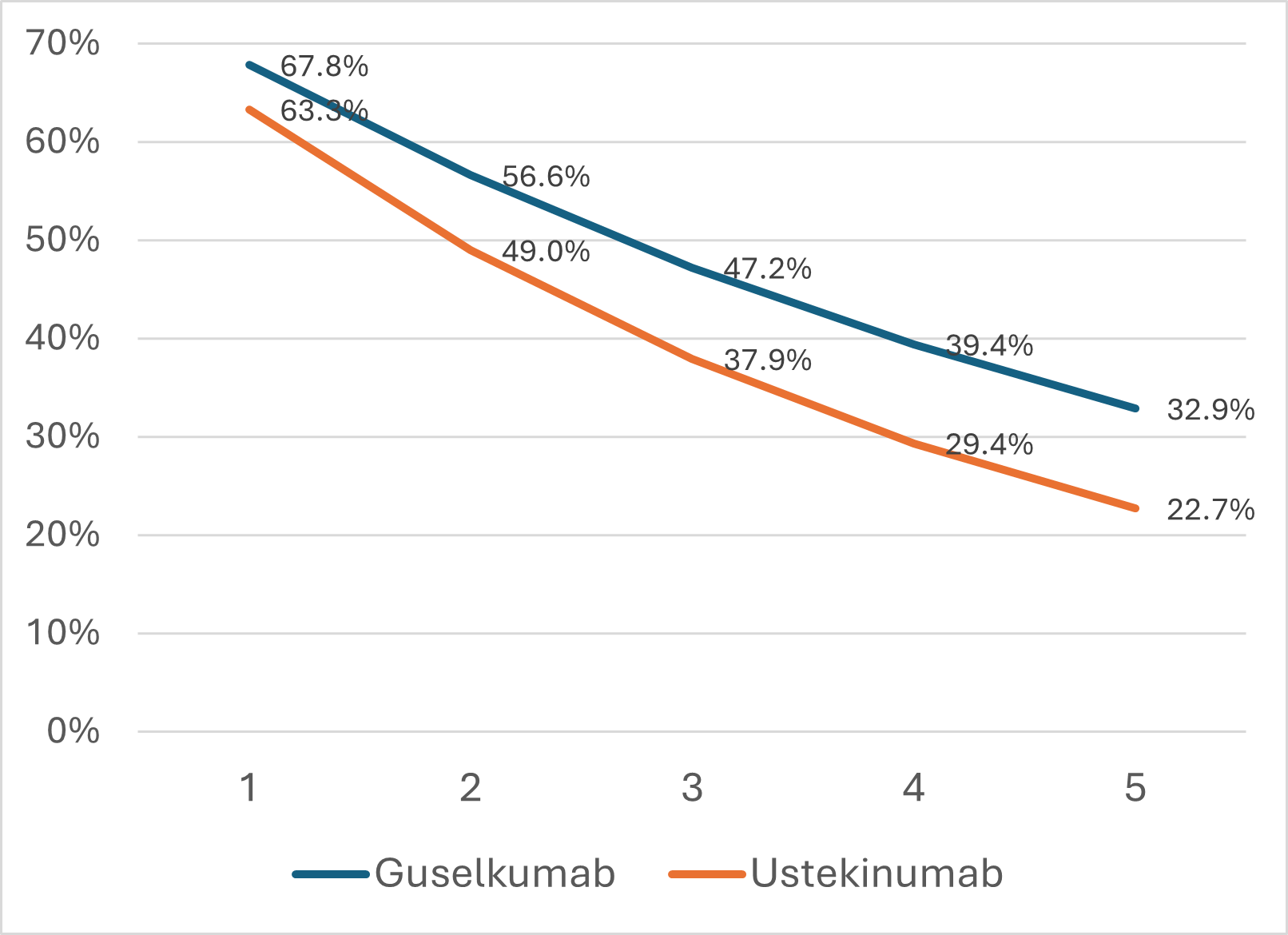
Figure: Figure. Predicted proportion of patients in clinical remission over 5 years

Figure: Table: Predicted proportion of patients maintaining clinical remission over 5 years, among those who achieved clinical remission in year 1
Disclosures:
Mariya Dimova: Johnson & Johnson – Employee.
Mi Jun Keng: Johnson & Johnson – Employee, Stock Options.
Feng Pan: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Dominik Naessens: Janssen Pharmaceutica N.V., – Employee.
Leonardo Salese: Johnson & Johnson – Employee.
Zijiang Yang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Sumesh Kachroo: Johnson & Johnson – Employee, Stock Options.
Mario Gomez Camacho: AstraZeneca – Employee. J&J Innovative Medicine – Employee, Stock Options.
Myrlene Sanon: Johnson & Johnson – Employee.
Mariya Dimova, 1, Mi Jun Keng, PhD2, Feng Pan, PhD3, Dominik Naessens, PharmD, MBA4, Leonardo Salese, MD5, Zijiang Yang, PhD5, Sumesh Kachroo, PhD6, Mario A. Gomez Camacho, MD, PhD, MSc, MBA7, Myrlene Sanon, MPH8. P3245 - Longer Sustained Remission in Crohn’s Disease With Guselkumab versus Ustekinumab Treatment: Projections From a Disease Model, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Janssen-Cilag, Issy-les-Moulineaux, Centre, France; 2Johnson & Johnson, Beerse, Antwerpen, Belgium; 3Johnson & Johnson, Raritan, NJ; 4Janssen Pharmaceutica N.V., Beerse, Brussels Hoofdstedelijk Gewest, Belgium; 5Johnson & Johnson, Spring House, PA; 6Johnson & Johnson, Horsham, PA; 7Johnson & Johnson, Philadelphia, PA; 8Johnson & Johnson, Metuchen, NJ
Introduction: Crohn’s disease (CD) is a progressive disease associated with reduced quality of life. Therapeutic goals and clinical guidelines have evolved to include endoscopic outcomes as a key prognostic parameter in disease management (STRIDE II). Guselkumab, a dual-acting IL-23 inhibitor, investigated in double-blind placebo-control, active comparator, Phase 3 trials, demonstrated superiority in 48-week efficacy to ustekinumab in achieving endoscopic response with 52.7% (p< 0.001) for guselkumab 200mg, and 47.9% (p=0.009) for guselkumab 100mg vs 37.1% for ustekinumab. To better understand the implication of endoscopic response on long-term remission, a disease model was developed to compare long-term disease clinical remission status for guselkumab and ustekinumab.
Methods: A state-transition model was developed to simulate long-term disease status change for patients with CD who initiate guselkumab or ustekinumab. In the model, patients can be in one of the three health states – clinical remission, clinical response without clinical remission, and active CD. For the first 48 weeks, the rates of clinical remission and clinical response are based on the GALAXI Phase 3 trials. After week 48, endoscopic response status based on galaxi 2-year long-term extension data were used to estimate the durability of clinical remission defined as those achieving clinical remission at each year over a 5-year period. Overtime, patients losing clinical remission or response will move to the active CD state.
Results: The model predicts the proportion of patients in clinical remission for guselkumab compared to ustekinumab at year two was 56.6% versus 49.7%, year three was 47.2% versus 37.9%, and year five was 32.9% versus 22.7% (Figure). The difference in proportion of patients attaining clinical remission with guselkumab compared to ustekinumab increases from 5% at year 1 (68% vs. 63%) to 10% at year 5 (33% vs. 23%). Model predicts over 5 years, among patients who achieved clinical remission at 1 year, 49% maintained clinical remission on guselkumab versus 36% on ustekinumab (Table).
Discussion: The model projections suggest treatment with guselkumab leads to better long-term outcomes for patients with CD, compared to ustekinumab. The results support the utility of endoscopic response as a predictor of long-term outcomes and suggests longer sustained clinical remission with guselkumab treatment versus ustekinumab.

Figure: Figure. Predicted proportion of patients in clinical remission over 5 years

Figure: Table: Predicted proportion of patients maintaining clinical remission over 5 years, among those who achieved clinical remission in year 1
Disclosures:
Mariya Dimova: Johnson & Johnson – Employee.
Mi Jun Keng: Johnson & Johnson – Employee, Stock Options.
Feng Pan: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Dominik Naessens: Janssen Pharmaceutica N.V., – Employee.
Leonardo Salese: Johnson & Johnson – Employee.
Zijiang Yang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Sumesh Kachroo: Johnson & Johnson – Employee, Stock Options.
Mario Gomez Camacho: AstraZeneca – Employee. J&J Innovative Medicine – Employee, Stock Options.
Myrlene Sanon: Johnson & Johnson – Employee.
Mariya Dimova, 1, Mi Jun Keng, PhD2, Feng Pan, PhD3, Dominik Naessens, PharmD, MBA4, Leonardo Salese, MD5, Zijiang Yang, PhD5, Sumesh Kachroo, PhD6, Mario A. Gomez Camacho, MD, PhD, MSc, MBA7, Myrlene Sanon, MPH8. P3245 - Longer Sustained Remission in Crohn’s Disease With Guselkumab versus Ustekinumab Treatment: Projections From a Disease Model, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
