Monday Poster Session
Category: IBD
P3241 - Patient-Reported Use of Concomitant Treatments for Crohn’s Disease Among Adults Treated With Risankizumab in Clinical Practice: Initial Results From the ASPIRE-CD Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aline Charabaty, MD, FACG (she/her/hers)
Johns Hopkins University School of Medicine
Washington, DC
Presenting Author(s)
Aline Charabaty, MD, FACG1, Bincy Abraham, MD, MS, FACG2, Jenny M. Griffith, PharmD3, Min Yang, MD4, Erin E. Cook, PhD5, Javier Zambrano, MD6, Julia Vishnevetsky, MPH7, Bruno Martins, PhD5, Laurie Keefer, PhD, FACG8
1Johns Hopkins University School of Medicine, Washington, DC; 2Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 3AbbVie Inc, North Chicago, IL; 4Analysis Group, Inc, Boston, MA; 5Analysis Group, Inc., Boston, MA; 6AbbVie Inc., North Chicago, IL; 7AbbVie, North Chicago, IL; 8Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Risankizumab (RZB) is an IL-23 p19 inhibitor biologic therapy approved for the treatment of moderately to severely active Crohn’s disease (CD). Here, we report initial results of the real-world impact of RZB on the use of concomitant treatments in patients with CD enrolled in the prospective, observational ASPIRE-CD study.
Methods: The ASPIRE-CD study included adult patients who initiated RZB for CD and enrolled in the RZB patient support program in the USA. Patients were asked to complete surveys before (baseline) and at weeks (wks) 2, 4, 12, and every 8 wks after the first RZB induction dose. Concomitant treatment was defined as using the treatment within the past two weeks for wk2 and wk4 surveys, and within the past four weeks for all other surveys. Patients were asked whether they were taking any concomitant treatments for CD in the last two to four weeks, including corticosteroids, over-the-counter (OTC) medications, and dietary adjustments (nutritional supplements, dietary restriction).
Results: Of the 286 patients who received the first induction RZB dose, 212 (76.0%) completed the wk20 survey. Most patients had prior biologic or Janus kinase inhibitor (JAKi) experience (75.2%) at baseline, with 45.5% having ≥ 2 prior biologics or JAKis. Patient-reported corticosteroid use reduced from 33.9% at baseline to 14.6% at wk20 (P < .001; Fig. 1A). The proportion of patients taking prescribed treatments other than steroids was low at baseline (5-aminosalicylates: 7.0%; immunosuppressants: 9.4%; antibiotics: 7.7%) and remained stable over 20 weeks. At wk20, the proportion of patients taking OTC treatments for CD was 53.8% vs 72.0% at baseline (P < .001; Fig. 1B). Patients taking OTC treatments for pain (acetaminophen, ibuprofen, or naproxen) decreased from 42.0% at baseline to 29.2% at wk20 (P < .01; Fig. 2A). Patients making dietary adjustments also reduced from 30.8% at baseline to 17.0% (P < .01; Fig. 1C). The proportion of patients taking vitamin or mineral supplementation decreased from baseline to wk20 (Fig. 2B), as did the proportion of patients restricting their diet (Fig. 2C).
Discussion: Initial results from this real-world study demonstrate that patients with moderately to severely active CD treated with RZB quickly (as early as wk4) reduced their use of concomitant corticosteroids, OTC medications to treat pain, and their need for diet restrictions and supplements. Future analyses of the ASPIRE-CD study will evaluate the long-term impact of RZB on concomitant treatment use.
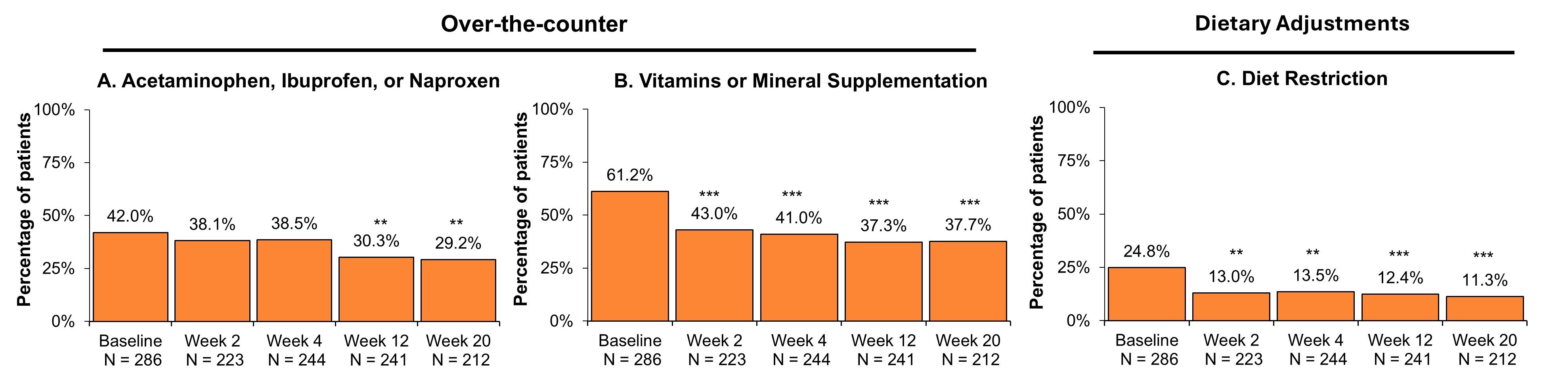
Figure: Figure 1. All Concomitant CD Treatments in Patients Initiating RZB Treatment.
Abbreviations: CD, Crohn’s disease; N, number; OTC, over-the-counter; RZB, risankizumab.
* P < .05, ** P < .01, *** P < .001.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables.
aCorticosteroids include oral prednisone, oral budesonide, and topical steroids.
bOTC treatments include methylcellulose, loperamide, acetaminophen, ibuprofen, naproxen, vitamins, and mineral supplementation.
cDietary adjustments include oral nutritional supplements and diet restriction (low fiber diet and low residual diet).
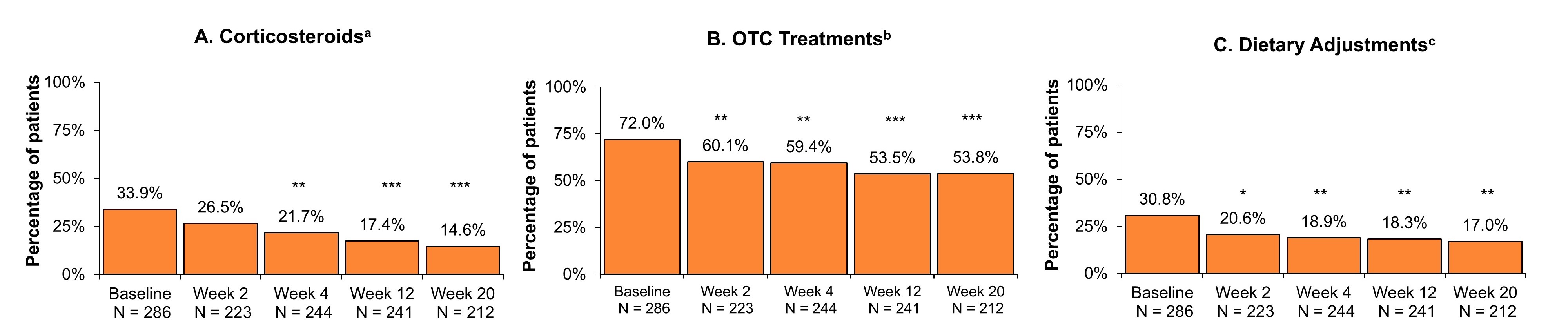
Figure: Figure 2. Concomitant OTC CD Treatments and Dietary Adjustments.
Abbreviations: CD, Crohn’s disease; N, number; OTC, over-the-counter; RZB, risankizumab.
** P < .01, *** P < .001.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables. Other OTCs evaluated included methylcellulose and loperamide; there was no change over time in these specific treatments. Other dietary adjustments included oral nutritional supplements; there was no change over time in this specific treatment category. Diet restrictions included low fiber diet and low residual diet.
Disclosures:
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Min Yang: Analysis Group, AbbVie Inc. – Consultant.
Erin Cook: Analysis Group, AbbVie Inc. – Consultant.
Javier Zambrano: AbbVie Inc. – Employee, Stock Options.
Julia Vishnevetsky: AbbVie Inc. – Employee, Stock Options.
Bruno Martins: Analysis Group, AbbVie Inc. – Consultant.
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Aline Charabaty, MD, FACG1, Bincy Abraham, MD, MS, FACG2, Jenny M. Griffith, PharmD3, Min Yang, MD4, Erin E. Cook, PhD5, Javier Zambrano, MD6, Julia Vishnevetsky, MPH7, Bruno Martins, PhD5, Laurie Keefer, PhD, FACG8. P3241 - Patient-Reported Use of Concomitant Treatments for Crohn’s Disease Among Adults Treated With Risankizumab in Clinical Practice: Initial Results From the ASPIRE-CD Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Johns Hopkins University School of Medicine, Washington, DC; 2Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 3AbbVie Inc, North Chicago, IL; 4Analysis Group, Inc, Boston, MA; 5Analysis Group, Inc., Boston, MA; 6AbbVie Inc., North Chicago, IL; 7AbbVie, North Chicago, IL; 8Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Risankizumab (RZB) is an IL-23 p19 inhibitor biologic therapy approved for the treatment of moderately to severely active Crohn’s disease (CD). Here, we report initial results of the real-world impact of RZB on the use of concomitant treatments in patients with CD enrolled in the prospective, observational ASPIRE-CD study.
Methods: The ASPIRE-CD study included adult patients who initiated RZB for CD and enrolled in the RZB patient support program in the USA. Patients were asked to complete surveys before (baseline) and at weeks (wks) 2, 4, 12, and every 8 wks after the first RZB induction dose. Concomitant treatment was defined as using the treatment within the past two weeks for wk2 and wk4 surveys, and within the past four weeks for all other surveys. Patients were asked whether they were taking any concomitant treatments for CD in the last two to four weeks, including corticosteroids, over-the-counter (OTC) medications, and dietary adjustments (nutritional supplements, dietary restriction).
Results: Of the 286 patients who received the first induction RZB dose, 212 (76.0%) completed the wk20 survey. Most patients had prior biologic or Janus kinase inhibitor (JAKi) experience (75.2%) at baseline, with 45.5% having ≥ 2 prior biologics or JAKis. Patient-reported corticosteroid use reduced from 33.9% at baseline to 14.6% at wk20 (P < .001; Fig. 1A). The proportion of patients taking prescribed treatments other than steroids was low at baseline (5-aminosalicylates: 7.0%; immunosuppressants: 9.4%; antibiotics: 7.7%) and remained stable over 20 weeks. At wk20, the proportion of patients taking OTC treatments for CD was 53.8% vs 72.0% at baseline (P < .001; Fig. 1B). Patients taking OTC treatments for pain (acetaminophen, ibuprofen, or naproxen) decreased from 42.0% at baseline to 29.2% at wk20 (P < .01; Fig. 2A). Patients making dietary adjustments also reduced from 30.8% at baseline to 17.0% (P < .01; Fig. 1C). The proportion of patients taking vitamin or mineral supplementation decreased from baseline to wk20 (Fig. 2B), as did the proportion of patients restricting their diet (Fig. 2C).
Discussion: Initial results from this real-world study demonstrate that patients with moderately to severely active CD treated with RZB quickly (as early as wk4) reduced their use of concomitant corticosteroids, OTC medications to treat pain, and their need for diet restrictions and supplements. Future analyses of the ASPIRE-CD study will evaluate the long-term impact of RZB on concomitant treatment use.

Figure: Figure 1. All Concomitant CD Treatments in Patients Initiating RZB Treatment.
Abbreviations: CD, Crohn’s disease; N, number; OTC, over-the-counter; RZB, risankizumab.
* P < .05, ** P < .01, *** P < .001.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables.
aCorticosteroids include oral prednisone, oral budesonide, and topical steroids.
bOTC treatments include methylcellulose, loperamide, acetaminophen, ibuprofen, naproxen, vitamins, and mineral supplementation.
cDietary adjustments include oral nutritional supplements and diet restriction (low fiber diet and low residual diet).

Figure: Figure 2. Concomitant OTC CD Treatments and Dietary Adjustments.
Abbreviations: CD, Crohn’s disease; N, number; OTC, over-the-counter; RZB, risankizumab.
** P < .01, *** P < .001.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables. Other OTCs evaluated included methylcellulose and loperamide; there was no change over time in these specific treatments. Other dietary adjustments included oral nutritional supplements; there was no change over time in this specific treatment category. Diet restrictions included low fiber diet and low residual diet.
Disclosures:
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Min Yang: Analysis Group, AbbVie Inc. – Consultant.
Erin Cook: Analysis Group, AbbVie Inc. – Consultant.
Javier Zambrano: AbbVie Inc. – Employee, Stock Options.
Julia Vishnevetsky: AbbVie Inc. – Employee, Stock Options.
Bruno Martins: Analysis Group, AbbVie Inc. – Consultant.
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Aline Charabaty, MD, FACG1, Bincy Abraham, MD, MS, FACG2, Jenny M. Griffith, PharmD3, Min Yang, MD4, Erin E. Cook, PhD5, Javier Zambrano, MD6, Julia Vishnevetsky, MPH7, Bruno Martins, PhD5, Laurie Keefer, PhD, FACG8. P3241 - Patient-Reported Use of Concomitant Treatments for Crohn’s Disease Among Adults Treated With Risankizumab in Clinical Practice: Initial Results From the ASPIRE-CD Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
