Monday Poster Session
Category: IBD
P3240 - Mirikizumab Demonstrates Durable and Sustained Maintenance of Endoscopic and Histologic Outcomes at 4 Years in Patients With Moderately-to-Severely Active Ulcerative Colitis: LUCENT-3 Open-Label Extension Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aline Charabaty, MD, FACG (she/her/hers)
Johns Hopkins University School of Medicine
Washington, DC
Presenting Author(s)
Simon Travis, 1, Bruce E. Sands, MD, MS, FACG2, Laurent Peyrin-Biroulet, MD, PhD3, Alissa Walsh, MBBS, PhD1, Bram Verstockt, MD, PhD4, Kim McGinnis, CPNP5, Anthony Keohane, MSc6, Deborah A.. Fisher, MD5, Guanglei Yu, PhD5, Aline Charabaty, MD, FACG7
1Translational Gastroenterology Unit, University of Oxford and Oxford Biomedical Research Centre, Oxford, UK, Oxford, England, United Kingdom; 2Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 3Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 4Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams-Brabant, Belgium; 5Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 6HaaPACS GmbH, Statistics Europe, Schriesheim, Germany, Timoleague, Cork, Ireland; 7Johns Hopkins University School of Medicine, Washington, DC
Introduction: Mirikizumab (MIRI), an anti-IL-23p19 monoclonal antibody, has previously shown maintained endoscopic and histologic remission with continuous treatment in patients with moderately-to-severely active ulcerative colitis (UC)1. We evaluated endoscopic and histologic outcomes after 4 years of continuous MIRI treatment.
Methods: The study designs for the continuous phase 3 LUCENT-1 (NCT03518086) 12-week induction, LUCENT-2 (NCT03524092) 40-week maintenance, and LUCENT-3 (NCT03519945) open-label long-term extension studies have been described2. This analysis focused on patients who were Week (W)52 MIRI maintenance clinical remitters. Durable maintenance of outcomes included endoscopic remission (ER), endoscopic normalization (EN), histologic improvement (HI), histologic remission (HR), histologic-endoscopic mucosal improvement (HEMI), histologic-endoscopic mucosal remission (HEMR), and alternate HEMR at 4 years. Sustained maintenance of ER, HI, HR, HEMI, and HEMR across 2, 3, and 4 years among the patients who achieved the respective endpoint at 1 year was also assessed. Discontinuations or missing data were handled by modified non-responder imputation (mNRI) and observed case (OC)2.
Results: The pre-treatment baseline characteristics for W52 clinical remitters (N=179): 59.2% were male, the mean (standard deviation (SD)) age was 43.8 (14.3) years. Mean (SD) of baseline modified Mayo Score was 6.6 (1.3) and 33.0% had prior biologic or tofacitinib failure. Figure 1 shows that after 4 years of continuous MIRI treatment, 81.3% of patients achieved ER, 67.9% achieved HR, 67.9% achieved HEMI, and 66.0% achieved HEMR (OC data). Figure 2 shows the percentages of patients who maintained outcomes at 2, 3, and 4 years among the patients who achieved the respective endpoint at 1 year, which were 70.0%, 57.7%, 50.0%, 53.8%, and 47.1% for ER, HI, HR, HEMI, and HEMR, respectively (OC data).
Discussion: In LUCENT-3, endoscopic and histologic outcomes, key components of comprehensive disease control, showed durable maintenance at 4 years of continuous mirikizumab treatment. These outcomes also showed sustained maintenance from 1 year across 2, 3, and 4 years. This is the only p19-targeted IL-23 antibody in UC showing endoscopic and histologic outcomes at 4 years.
1Sands, B et al. The American Journal of Gastroenterology 2024: 119(10S):p S1042-S1043
2Sands, B et al. Inflammatory Bowel Diseases 2024; izae253
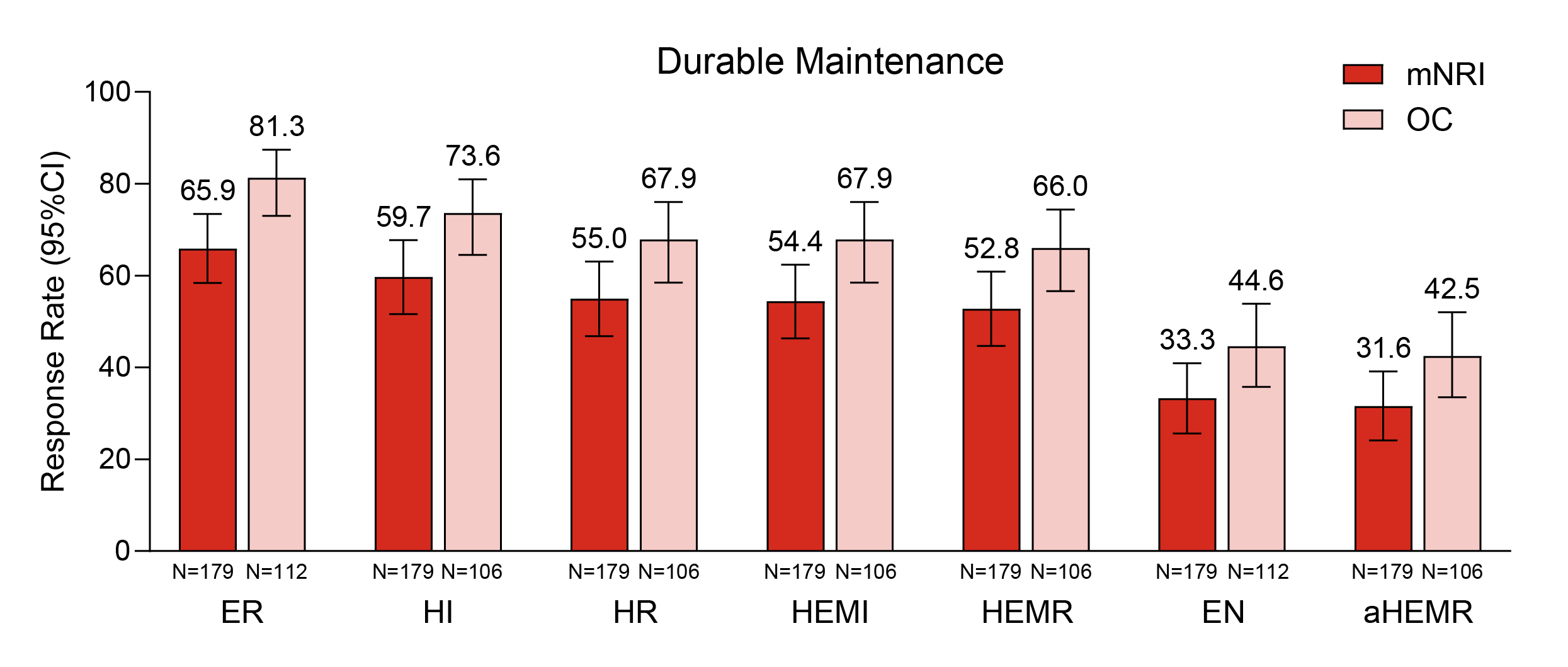
Figure: Figure 1: Proportion of patients who achieved endoscopic and histologic outcomes at 4 years of MIRI treatment among clinical remitters at W52.
The modified intent-to-treat population was used. CIs were constructed using Wilson method without continuity correction for OC; percentages of response were calculated, and CIs were constructed using Rubin's Rules to combine multiple imputation datasets for mNRI.
ER: Mayo ES = 0 or 1 [excluding friability]; HI: Geboes score ≤3.1; HR: Geboes score ≤2B.0; HEMI: HI and ER: Geboes score ≤3.1 plus Mayo ES = 0 or 1 [excluding friability]; HEMR: HR and ER: Geboes score ≤2B.0 plus Mayo ES = 0 or 1 [excluding friability]; EN: Mayo ES = 0; aHEMR: HR and EN: Geboes score ≤2B.0 plus Mayo ES = 0. Mayo Scoring System to Assess Activity of Ulcerative Colitis Symptoms. Copyright Mayo Clinic.
aHEMR=alternate HEMR; CI=confidence interval; EN=endoscopic normalization; ER=endoscopic remission; ES=endoscopic score; HEMI=histologic-endoscopic mucosal improvement; HEMR=histologic-endoscopic mucosal remission; HI=histologic improvement; HR=histologic remission; MMS=Modified Mayo Score; mNRI=modified non-responder imputation; N=number of patients; OC=observed case; W=week.
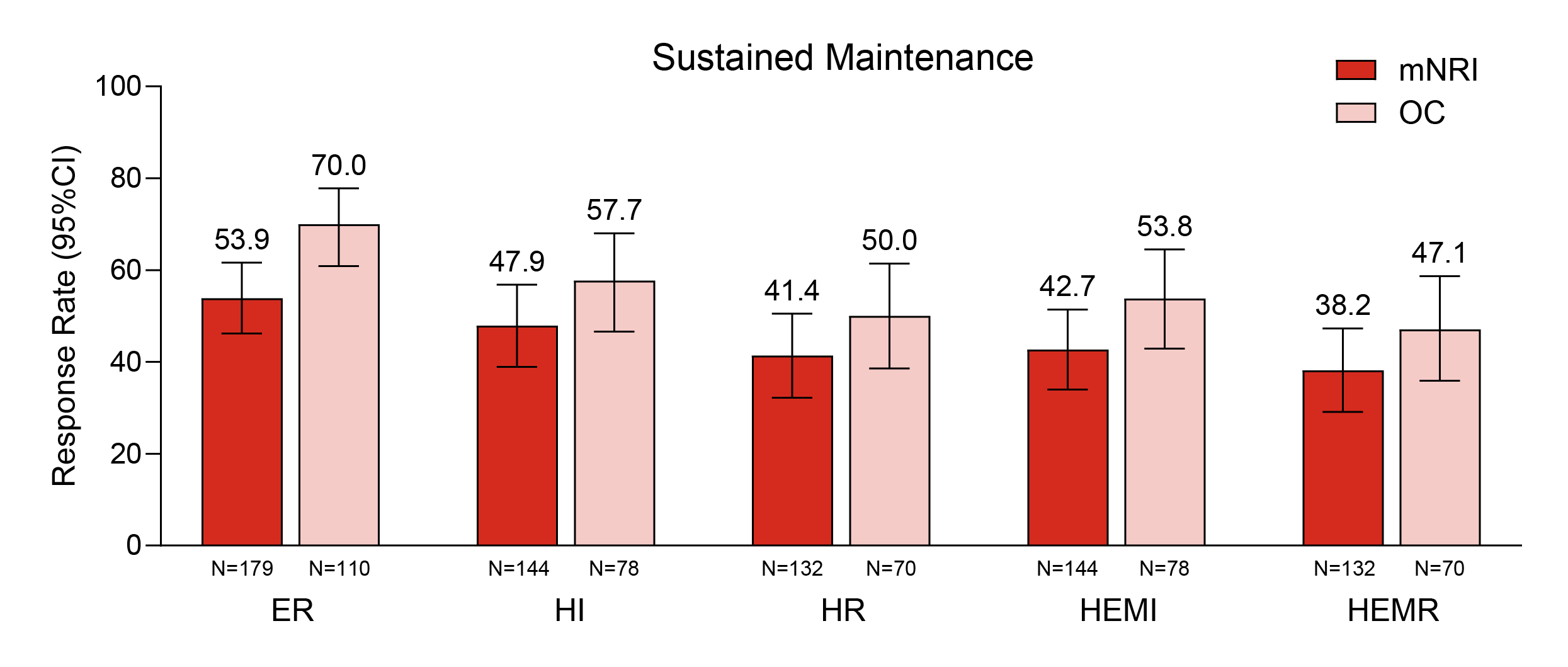
Figure: Figure 2: Proportion of patients who maintained endoscopic and histologic outcomes across 2, 3, and 4 years of MIRI treatment among clinical remitters at W52.
Data shows the proportion of patients who achieved outcomes at 1 year (52 weeks continuous treatment) and maintained them at 2 years (104 weeks), 3 years (152 weeks), and 4 years (212 weeks). Only patients who achieved the endpoint at 1 year were included in the analysis. The modified intent-to-treat population was used. CIs were constructed using Wilson method without continuity correction for OC; percentages of response were calculated, and CIs were constructed using Rubin's Rules to combine multiple imputation datasets for mNRI.
ER: Mayo ES = 0 or 1 [excluding friability]; HI: Geboes score ≤3.1; HR: Geboes score ≤2B.0; HEMI: HI and ER: Geboes score ≤3.1 plus Mayo ES = 0 or 1 [excluding friability]; HEMR: HR and ER: Geboes score ≤2B.0 plus Mayo ES = 0 or 1 [excluding friability]. Mayo Scoring System to Assess Activity of Ulcerative Colitis Symptoms. Copyright Mayo Clinic.
CI=confidence interval; ER=endoscopic remission; ES=endoscopic score; HEMI=histologic-endoscopic mucosal improvement; HEMR=histologic-endoscopic mucosal remission; HI=histologic improvement; HR=histologic remission; MMS=Modified Mayo Score; mNRI=modified non-responder imputation; N=number of patients who achieved the endpoint at 1 year; OC=observed case; W=week.
Disclosures:
Simon Travis: AbbVie – Grant/Research Support, Honoraria, Travel expenses. Alimentiv – Independent Contractor. Bioclinica – Consultant. Bristol Myers Squibb – Consultant. Cosmo – Consultant. Dova Health Intelligence – Consultant, Stock Options. ECCO Health Care – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support, Honoraria. Equillium – Consultant. Ferring – Consultant, Honoraria, Travel expenses. Genentech – Consultant. GlaxoSmithKline – Consultant. Immunocore – Consultant. Immunometabolism – Consultant. Janssen – Consultant. Mestag – Consultant. MSD – Consultant. Norman Collisson Foundation – Grant/Research Support. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support, Travel expenses. Protagonist – Consultant. Roche – Consultant. Satisfai – Consultant. Sorriso – Consultant. Spyre – Consultant. Takeda – Consultant, Honoraria, Travel expenses. The Helmsley Trust – Grant/Research Support. the International Organization for the Study of Inflammatory Bowel Diseases – Grant/Research Support. UCB – Consultant, Grant/Research Support. UK–India Education and Research Initiative – Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Alissa Walsh: Abbvie – Consultant, Speakers Bureau. Bristol-Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Bram Verstockt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Agomab – Speakers Bureau. Alfasifgma – Advisory Committee/Board Member, Speakers Bureau. Alimentiv – Consultant. Anaptys Bio – Advisory Committee/Board Member. Applied Strategic – Advisory Committee/Board Member. Astrazeneca – Advisory Committee/Board Member. Atheneum – Advisory Committee/Board Member. BenevolentAI – Advisory Committee/Board Member. Biogen – Speakers Bureau. Biora Thearapeutics – Advisory Committee/Board Member, Grant/Research Support. Boxer Capital – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celltrion – Grant/Research Support, Speakers Bureau. Domain Therapeutics – Advisory Committee/Board Member. Eli Lily – Advisory Committee/Board Member, Speakers Bureau. Falk – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Advisory Committee/Board Member, Speakers Bureau. Inotrem – Advisory Committee/Board Member. Johnson and Johnson – Advisory Committee/Board Member, Speakers Bureau. Landos – Grant/Research Support. Merck – Advisory Committee/Board Member. Mirador Therapeutics – Advisory Committee/Board Member. Nxera – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Sandoz – Speakers Bureau. Sanofi – Advisory Committee/Board Member, Grant/Research Support. Santa Ana Bio – Advisory Committee/Board Member. Sapphire Therapeutics – Advisory Committee/Board Member. Sosei Heptares – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau. Thethis Pharma – Stock Options. Tillots Pharma – Advisory Committee/Board Member, Speakers Bureau. Truvion – Speakers Bureau. Vagustim – Stock Options. Viatris – Speakers Bureau.
Kim McGinnis: Eli Lilly – Employee, Stock-publicly held company(excluding mutual/index funds).
Anthony Keohane: Eli Lilly and Company – Independent Contractor.
Deborah Fisher: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Simon Travis, 1, Bruce E. Sands, MD, MS, FACG2, Laurent Peyrin-Biroulet, MD, PhD3, Alissa Walsh, MBBS, PhD1, Bram Verstockt, MD, PhD4, Kim McGinnis, CPNP5, Anthony Keohane, MSc6, Deborah A.. Fisher, MD5, Guanglei Yu, PhD5, Aline Charabaty, MD, FACG7. P3240 - Mirikizumab Demonstrates Durable and Sustained Maintenance of Endoscopic and Histologic Outcomes at 4 Years in Patients With Moderately-to-Severely Active Ulcerative Colitis: LUCENT-3 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Translational Gastroenterology Unit, University of Oxford and Oxford Biomedical Research Centre, Oxford, UK, Oxford, England, United Kingdom; 2Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 3Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 4Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams-Brabant, Belgium; 5Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 6HaaPACS GmbH, Statistics Europe, Schriesheim, Germany, Timoleague, Cork, Ireland; 7Johns Hopkins University School of Medicine, Washington, DC
Introduction: Mirikizumab (MIRI), an anti-IL-23p19 monoclonal antibody, has previously shown maintained endoscopic and histologic remission with continuous treatment in patients with moderately-to-severely active ulcerative colitis (UC)1. We evaluated endoscopic and histologic outcomes after 4 years of continuous MIRI treatment.
Methods: The study designs for the continuous phase 3 LUCENT-1 (NCT03518086) 12-week induction, LUCENT-2 (NCT03524092) 40-week maintenance, and LUCENT-3 (NCT03519945) open-label long-term extension studies have been described2. This analysis focused on patients who were Week (W)52 MIRI maintenance clinical remitters. Durable maintenance of outcomes included endoscopic remission (ER), endoscopic normalization (EN), histologic improvement (HI), histologic remission (HR), histologic-endoscopic mucosal improvement (HEMI), histologic-endoscopic mucosal remission (HEMR), and alternate HEMR at 4 years. Sustained maintenance of ER, HI, HR, HEMI, and HEMR across 2, 3, and 4 years among the patients who achieved the respective endpoint at 1 year was also assessed. Discontinuations or missing data were handled by modified non-responder imputation (mNRI) and observed case (OC)2.
Results: The pre-treatment baseline characteristics for W52 clinical remitters (N=179): 59.2% were male, the mean (standard deviation (SD)) age was 43.8 (14.3) years. Mean (SD) of baseline modified Mayo Score was 6.6 (1.3) and 33.0% had prior biologic or tofacitinib failure. Figure 1 shows that after 4 years of continuous MIRI treatment, 81.3% of patients achieved ER, 67.9% achieved HR, 67.9% achieved HEMI, and 66.0% achieved HEMR (OC data). Figure 2 shows the percentages of patients who maintained outcomes at 2, 3, and 4 years among the patients who achieved the respective endpoint at 1 year, which were 70.0%, 57.7%, 50.0%, 53.8%, and 47.1% for ER, HI, HR, HEMI, and HEMR, respectively (OC data).
Discussion: In LUCENT-3, endoscopic and histologic outcomes, key components of comprehensive disease control, showed durable maintenance at 4 years of continuous mirikizumab treatment. These outcomes also showed sustained maintenance from 1 year across 2, 3, and 4 years. This is the only p19-targeted IL-23 antibody in UC showing endoscopic and histologic outcomes at 4 years.
1Sands, B et al. The American Journal of Gastroenterology 2024: 119(10S):p S1042-S1043
2Sands, B et al. Inflammatory Bowel Diseases 2024; izae253

Figure: Figure 1: Proportion of patients who achieved endoscopic and histologic outcomes at 4 years of MIRI treatment among clinical remitters at W52.
The modified intent-to-treat population was used. CIs were constructed using Wilson method without continuity correction for OC; percentages of response were calculated, and CIs were constructed using Rubin's Rules to combine multiple imputation datasets for mNRI.
ER: Mayo ES = 0 or 1 [excluding friability]; HI: Geboes score ≤3.1; HR: Geboes score ≤2B.0; HEMI: HI and ER: Geboes score ≤3.1 plus Mayo ES = 0 or 1 [excluding friability]; HEMR: HR and ER: Geboes score ≤2B.0 plus Mayo ES = 0 or 1 [excluding friability]; EN: Mayo ES = 0; aHEMR: HR and EN: Geboes score ≤2B.0 plus Mayo ES = 0. Mayo Scoring System to Assess Activity of Ulcerative Colitis Symptoms. Copyright Mayo Clinic.
aHEMR=alternate HEMR; CI=confidence interval; EN=endoscopic normalization; ER=endoscopic remission; ES=endoscopic score; HEMI=histologic-endoscopic mucosal improvement; HEMR=histologic-endoscopic mucosal remission; HI=histologic improvement; HR=histologic remission; MMS=Modified Mayo Score; mNRI=modified non-responder imputation; N=number of patients; OC=observed case; W=week.

Figure: Figure 2: Proportion of patients who maintained endoscopic and histologic outcomes across 2, 3, and 4 years of MIRI treatment among clinical remitters at W52.
Data shows the proportion of patients who achieved outcomes at 1 year (52 weeks continuous treatment) and maintained them at 2 years (104 weeks), 3 years (152 weeks), and 4 years (212 weeks). Only patients who achieved the endpoint at 1 year were included in the analysis. The modified intent-to-treat population was used. CIs were constructed using Wilson method without continuity correction for OC; percentages of response were calculated, and CIs were constructed using Rubin's Rules to combine multiple imputation datasets for mNRI.
ER: Mayo ES = 0 or 1 [excluding friability]; HI: Geboes score ≤3.1; HR: Geboes score ≤2B.0; HEMI: HI and ER: Geboes score ≤3.1 plus Mayo ES = 0 or 1 [excluding friability]; HEMR: HR and ER: Geboes score ≤2B.0 plus Mayo ES = 0 or 1 [excluding friability]. Mayo Scoring System to Assess Activity of Ulcerative Colitis Symptoms. Copyright Mayo Clinic.
CI=confidence interval; ER=endoscopic remission; ES=endoscopic score; HEMI=histologic-endoscopic mucosal improvement; HEMR=histologic-endoscopic mucosal remission; HI=histologic improvement; HR=histologic remission; MMS=Modified Mayo Score; mNRI=modified non-responder imputation; N=number of patients who achieved the endpoint at 1 year; OC=observed case; W=week.
Disclosures:
Simon Travis: AbbVie – Grant/Research Support, Honoraria, Travel expenses. Alimentiv – Independent Contractor. Bioclinica – Consultant. Bristol Myers Squibb – Consultant. Cosmo – Consultant. Dova Health Intelligence – Consultant, Stock Options. ECCO Health Care – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support, Honoraria. Equillium – Consultant. Ferring – Consultant, Honoraria, Travel expenses. Genentech – Consultant. GlaxoSmithKline – Consultant. Immunocore – Consultant. Immunometabolism – Consultant. Janssen – Consultant. Mestag – Consultant. MSD – Consultant. Norman Collisson Foundation – Grant/Research Support. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support, Travel expenses. Protagonist – Consultant. Roche – Consultant. Satisfai – Consultant. Sorriso – Consultant. Spyre – Consultant. Takeda – Consultant, Honoraria, Travel expenses. The Helmsley Trust – Grant/Research Support. the International Organization for the Study of Inflammatory Bowel Diseases – Grant/Research Support. UCB – Consultant, Grant/Research Support. UK–India Education and Research Initiative – Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Alissa Walsh: Abbvie – Consultant, Speakers Bureau. Bristol-Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Bram Verstockt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Agomab – Speakers Bureau. Alfasifgma – Advisory Committee/Board Member, Speakers Bureau. Alimentiv – Consultant. Anaptys Bio – Advisory Committee/Board Member. Applied Strategic – Advisory Committee/Board Member. Astrazeneca – Advisory Committee/Board Member. Atheneum – Advisory Committee/Board Member. BenevolentAI – Advisory Committee/Board Member. Biogen – Speakers Bureau. Biora Thearapeutics – Advisory Committee/Board Member, Grant/Research Support. Boxer Capital – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celltrion – Grant/Research Support, Speakers Bureau. Domain Therapeutics – Advisory Committee/Board Member. Eli Lily – Advisory Committee/Board Member, Speakers Bureau. Falk – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Advisory Committee/Board Member, Speakers Bureau. Inotrem – Advisory Committee/Board Member. Johnson and Johnson – Advisory Committee/Board Member, Speakers Bureau. Landos – Grant/Research Support. Merck – Advisory Committee/Board Member. Mirador Therapeutics – Advisory Committee/Board Member. Nxera – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Sandoz – Speakers Bureau. Sanofi – Advisory Committee/Board Member, Grant/Research Support. Santa Ana Bio – Advisory Committee/Board Member. Sapphire Therapeutics – Advisory Committee/Board Member. Sosei Heptares – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau. Thethis Pharma – Stock Options. Tillots Pharma – Advisory Committee/Board Member, Speakers Bureau. Truvion – Speakers Bureau. Vagustim – Stock Options. Viatris – Speakers Bureau.
Kim McGinnis: Eli Lilly – Employee, Stock-publicly held company(excluding mutual/index funds).
Anthony Keohane: Eli Lilly and Company – Independent Contractor.
Deborah Fisher: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Simon Travis, 1, Bruce E. Sands, MD, MS, FACG2, Laurent Peyrin-Biroulet, MD, PhD3, Alissa Walsh, MBBS, PhD1, Bram Verstockt, MD, PhD4, Kim McGinnis, CPNP5, Anthony Keohane, MSc6, Deborah A.. Fisher, MD5, Guanglei Yu, PhD5, Aline Charabaty, MD, FACG7. P3240 - Mirikizumab Demonstrates Durable and Sustained Maintenance of Endoscopic and Histologic Outcomes at 4 Years in Patients With Moderately-to-Severely Active Ulcerative Colitis: LUCENT-3 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
