Monday Poster Session
Category: IBD
P3237 - Role of Mirikizumab in Inducing and Maintaining Remission in Crohn’s Disease: A Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Zoha Shahzad, MD (she/her/hers)
One Brooklyn Health-Brookdale University Hospital Medical Center
Brooklyn, NY
Presenting Author(s)
Zoha Shahzad, MD1, Osama Ijaz, MD2, Hameed Ullah, MD3, Ahmad Zain, MBBS4, Nouman Shafique, MD5, Badr Ilmaguook, MD6, Muhammad Abdul Moiz, MBBS7, Ishrat Fatima, MBBS8, Mah Rukh, MBBS9, Zahra Yasmeen, MBBS10
1One Brooklyn Health-Brookdale University Hospital Medical Center, Brooklyn, NY; 2SSM Health St. Mary's Hospital - St. Louis, Richmond Heights, MO; 3St Luke’s Hospital, Chesterfield, Chesterfield, MO; 4Parkview Medical Center, Pueblo, CO; 5AdventHealth Orlando, Orlando, FL; 6NYC Health + Hospitals/Woodhull, Brooklyn, NY; 7Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 8Fatima Jinnah Medical University, Lahore, Punjab, Pakistan; 9Faisalabad Medical University, Faisalabad, Punjab, Pakistan; 10Aga Khan University, Karachi, Sindh, Pakistan
Introduction: Crohn disease (CD) often leads to steroid dependence or treatment resistance without timely biologic intervention. Mirikizumab, a humanized monoclonal antibody targeting IL-23p19, has demonstrated efficacy in ulcerative colitis. This meta-analysis assesses its therapeutic potential in patients with moderate to severe Crohn disease.
Methods: We systematically searched PubMed, MEDLINE, SCOPUS, Google Scholar, and Cochrane CENTRAL through May 2025 for randomized controlled trials (RCTs) evaluating Mirikizumab 1000 mg in Crohn disease. Primary outcomes included endoscopic response (≥50% reduction in Simple Endoscopic Score for Crohn Disease [SES-CD]) or histologic response ( >50% reduction in Robarts Histopathology Index [RHI] or Global Histologic Activity Score [GHAS]) at weeks 12 and 52, along with endoscopic or histologic remission (based on SES-CD and absence of mucosal neutrophils and epithelial damage). Secondary outcomes were patient-reported (PRO) clinical response at week 12 (≥30% decrease in abdominal pain and/or stool frequency) and Crohn’s Disease Activity Index [CDAI] remission (CDAI < 150) at week 52. A random-effects meta-analysis was conducted, reporting pooled risk ratios (RR) with 95% confidence intervals (CI).
Results: Three RCTs involving 1,511 participants were included. Mirikizumab significantly outperformed placebo in achieving endoscopic/histologic response at both 12 weeks (RR 2.91, 95% CI: 1.86–4.54; P< 0.00001; I²=9%) and 52 weeks (RR 2.80, 95% CI: 1.15–6.80; P=0.02; I²=83%). It also showed superior rates of remission at 12 weeks (RR 7.41, 95% CI: 2.43–22.65; P=0.0004; I²=0%). However, remission rates at 52 weeks were comparable to placebo (RR 1.40, 95% CI: 0.76–2.58; P=0.27; I²=0%). For secondary outcomes, Mirikizumab was more effective in achieving PRO clinical response at 12 weeks (RR 6.16, 95% CI: 4.02–9.43; P< 0.00001; I²=0%) but did not significantly improve CDAI remission at 52 weeks (RR 1.74, 95% CI: 0.44–6.86; P=0.43; I²=92%).
Discussion: Mirikizumab demonstrates promising efficacy in both short- and long-term management of moderate-to-severe Crohn disease. However, further comparative studies are needed to evaluate its effectiveness and safety against other available biologic therapies.
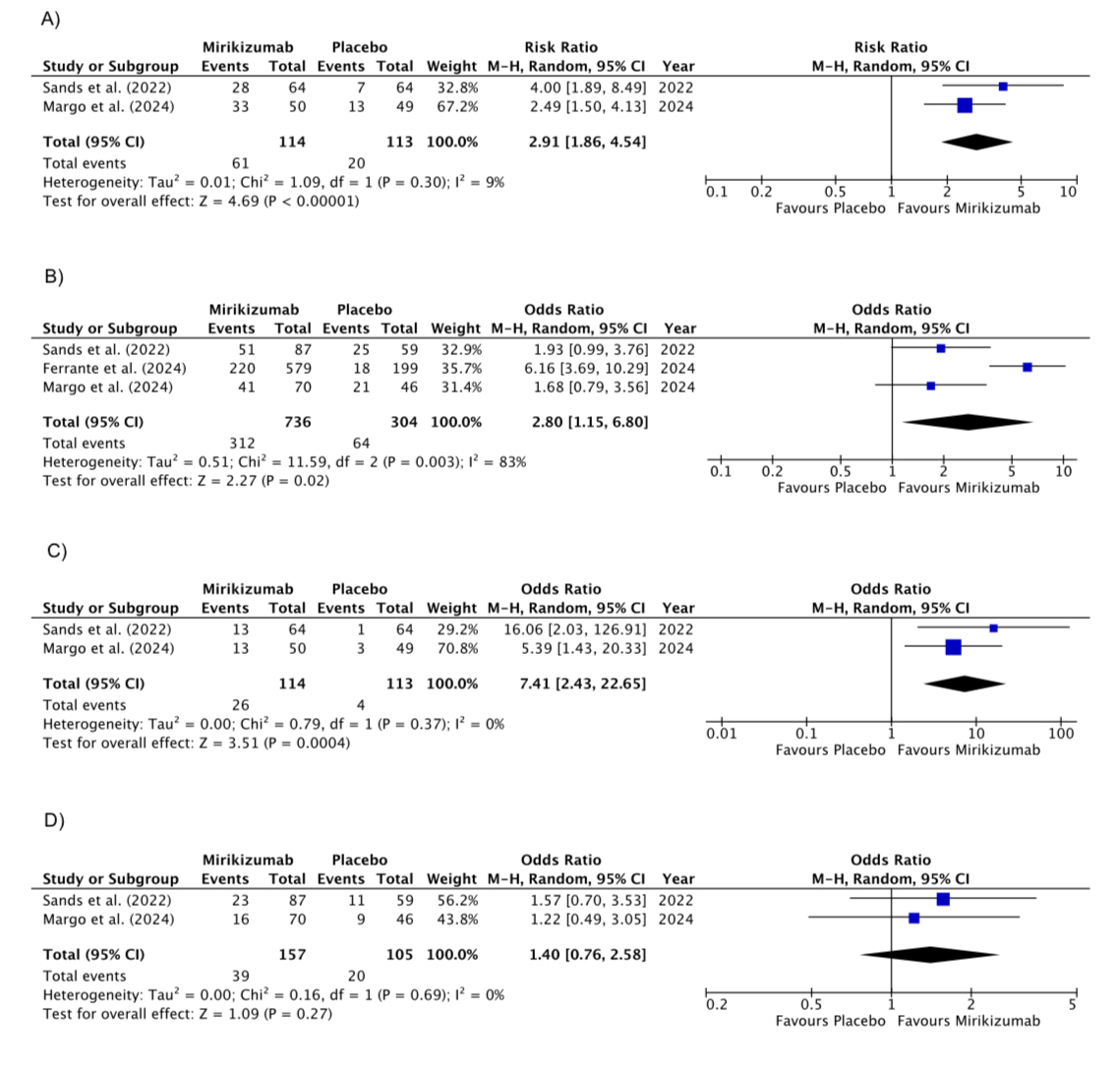
Figure: Forest plots comparing Mirikizumab versus placebo for: (A) endoscopic or histologic response at 12 weeks; (B) endoscopic or histologic response at 52 weeks; (C) endoscopic or histologic remission at 12 weeks; and (D) endoscopic or histologic remission at 52 weeks.
Disclosures:
Zoha Shahzad indicated no relevant financial relationships.
Osama Ijaz indicated no relevant financial relationships.
Hameed Ullah indicated no relevant financial relationships.
Ahmad Zain indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Badr Ilmaguook indicated no relevant financial relationships.
Muhammad Abdul Moiz indicated no relevant financial relationships.
Ishrat Fatima indicated no relevant financial relationships.
Mah Rukh indicated no relevant financial relationships.
Zahra Yasmeen indicated no relevant financial relationships.
Zoha Shahzad, MD1, Osama Ijaz, MD2, Hameed Ullah, MD3, Ahmad Zain, MBBS4, Nouman Shafique, MD5, Badr Ilmaguook, MD6, Muhammad Abdul Moiz, MBBS7, Ishrat Fatima, MBBS8, Mah Rukh, MBBS9, Zahra Yasmeen, MBBS10. P3237 - Role of Mirikizumab in Inducing and Maintaining Remission in Crohn’s Disease: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1One Brooklyn Health-Brookdale University Hospital Medical Center, Brooklyn, NY; 2SSM Health St. Mary's Hospital - St. Louis, Richmond Heights, MO; 3St Luke’s Hospital, Chesterfield, Chesterfield, MO; 4Parkview Medical Center, Pueblo, CO; 5AdventHealth Orlando, Orlando, FL; 6NYC Health + Hospitals/Woodhull, Brooklyn, NY; 7Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 8Fatima Jinnah Medical University, Lahore, Punjab, Pakistan; 9Faisalabad Medical University, Faisalabad, Punjab, Pakistan; 10Aga Khan University, Karachi, Sindh, Pakistan
Introduction: Crohn disease (CD) often leads to steroid dependence or treatment resistance without timely biologic intervention. Mirikizumab, a humanized monoclonal antibody targeting IL-23p19, has demonstrated efficacy in ulcerative colitis. This meta-analysis assesses its therapeutic potential in patients with moderate to severe Crohn disease.
Methods: We systematically searched PubMed, MEDLINE, SCOPUS, Google Scholar, and Cochrane CENTRAL through May 2025 for randomized controlled trials (RCTs) evaluating Mirikizumab 1000 mg in Crohn disease. Primary outcomes included endoscopic response (≥50% reduction in Simple Endoscopic Score for Crohn Disease [SES-CD]) or histologic response ( >50% reduction in Robarts Histopathology Index [RHI] or Global Histologic Activity Score [GHAS]) at weeks 12 and 52, along with endoscopic or histologic remission (based on SES-CD and absence of mucosal neutrophils and epithelial damage). Secondary outcomes were patient-reported (PRO) clinical response at week 12 (≥30% decrease in abdominal pain and/or stool frequency) and Crohn’s Disease Activity Index [CDAI] remission (CDAI < 150) at week 52. A random-effects meta-analysis was conducted, reporting pooled risk ratios (RR) with 95% confidence intervals (CI).
Results: Three RCTs involving 1,511 participants were included. Mirikizumab significantly outperformed placebo in achieving endoscopic/histologic response at both 12 weeks (RR 2.91, 95% CI: 1.86–4.54; P< 0.00001; I²=9%) and 52 weeks (RR 2.80, 95% CI: 1.15–6.80; P=0.02; I²=83%). It also showed superior rates of remission at 12 weeks (RR 7.41, 95% CI: 2.43–22.65; P=0.0004; I²=0%). However, remission rates at 52 weeks were comparable to placebo (RR 1.40, 95% CI: 0.76–2.58; P=0.27; I²=0%). For secondary outcomes, Mirikizumab was more effective in achieving PRO clinical response at 12 weeks (RR 6.16, 95% CI: 4.02–9.43; P< 0.00001; I²=0%) but did not significantly improve CDAI remission at 52 weeks (RR 1.74, 95% CI: 0.44–6.86; P=0.43; I²=92%).
Discussion: Mirikizumab demonstrates promising efficacy in both short- and long-term management of moderate-to-severe Crohn disease. However, further comparative studies are needed to evaluate its effectiveness and safety against other available biologic therapies.

Figure: Forest plots comparing Mirikizumab versus placebo for: (A) endoscopic or histologic response at 12 weeks; (B) endoscopic or histologic response at 52 weeks; (C) endoscopic or histologic remission at 12 weeks; and (D) endoscopic or histologic remission at 52 weeks.
Disclosures:
Zoha Shahzad indicated no relevant financial relationships.
Osama Ijaz indicated no relevant financial relationships.
Hameed Ullah indicated no relevant financial relationships.
Ahmad Zain indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Badr Ilmaguook indicated no relevant financial relationships.
Muhammad Abdul Moiz indicated no relevant financial relationships.
Ishrat Fatima indicated no relevant financial relationships.
Mah Rukh indicated no relevant financial relationships.
Zahra Yasmeen indicated no relevant financial relationships.
Zoha Shahzad, MD1, Osama Ijaz, MD2, Hameed Ullah, MD3, Ahmad Zain, MBBS4, Nouman Shafique, MD5, Badr Ilmaguook, MD6, Muhammad Abdul Moiz, MBBS7, Ishrat Fatima, MBBS8, Mah Rukh, MBBS9, Zahra Yasmeen, MBBS10. P3237 - Role of Mirikizumab in Inducing and Maintaining Remission in Crohn’s Disease: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
