Monday Poster Session
Category: IBD
P3196 - Efficacy of Biologics and Small Molecules for Induction of Response and Remission in Fistulizing Crohn's Disease: A Network Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Shahryar Khan, MD
University of Kansas Medical Center
Kansas City, KS
Presenting Author(s)
Award: ACG Presidential Poster Award
Shahryar Khan, MD1, Noor Ehsan, MD2, Manav Nayeni, BMSc3, Ryan Nazari, BS3, Azzah Muhammad Hayat, MD4, Ahmad Khan, 4, Hameed Ullah, MD5, Asad Ur Rahman, MD6, Shadi Hamdeh, MD, FACG1
1University of Kansas Medical Center, Kansas City, KS; 2Gulab Devi Teaching Hospital, Lahore, Punjab, Pakistan; 3Kansas City University, Kansas City, MO; 4Khyber Medical University, Peshawar, North-West Frontier, Pakistan; 5St. Luke's Hospital, Chesterfield, MO; 6Cleveland Clinic Florida, Weston, FL
Introduction: Fistulizing Crohn’s disease (FCD) constitutes a complex and aggressive disease phenotype characterized by penetrating complications and substantial morbidity. The induction of clinical remission and fistula response remains a critical therapeutic objective; however, comparative efficacy data across the available advanced therapies are limited. This network meta-analysis (NMA) aimed to compare the efficacy of biologics and small molecules in inducing initial clinical remission and response in patients with FCD.
Methods: We conducted a systematic review and NMA of RCTs, and only studies reporting clinical outcomes with at least six months of follow-up were included. Fistula remission was defined as complete closure of all external draining fistulas and response as a ≥50% reduction in the number of draining fistulas from baseline without new fistula formation. A frequentist random-effects NMA was performed using the netmeta package in R to estimate the odds ratios and P-scores for treatment ranking.
Results: Thirteen RCTs were included to evaluate the efficacy of 14 advanced therapies. The predominant phenotype observed among the study participants was perianal FCD. For remission, Adalimumab plus Ciprofloxacin (OR 6.59; 95% CI 1.27-34.2), Infliximab 5 mg/kg (OR 3.83; 95% CI 1.82-8.05), and Upadacitinib (OR 5.67; 95% CI 1.11-29.03) significantly increased the odds of achieving complete fistula closure compared to placebo (Fig.1, Fig.2). Among the evaluated treatments, Adalimumab plus Ciprofloxacin ranked highest for induction of remission, followed by Upadacitinib (Fig.3). Regarding response, Infliximab plus Ciprofloxacin (OR 25.7; 95% CI 2.43-273), Infliximab 5 mg/kg (OR 6.04; 95% CI 1.53-23.8), Upadacitinib (OR 13.7; 95% CI 1.42-133.2), and Ustekinumab (OR 2.50; 95% CI 1.08-5.76) significantly improved clinical outcomes compared to placebo (Fig.4). Infliximab plus Ciprofloxacin ranked the highest for response, followed by Upadacitinib. Vedolizumab and Filgotinib did not demonstrate superiority over placebo in either remission or response.
Discussion: This analysis suggests that combination therapies, particularly those involving anti-TNF agents with antibiotics and novel agents, such as Upadacitinib, are associated with the most favorable induction of clinical remission and response in FCD. However, data on newer IL-23 inhibitors remain limited, underscoring the need for additional studies and head-to-head trials to better define their roles and guide optimal treatment selection.
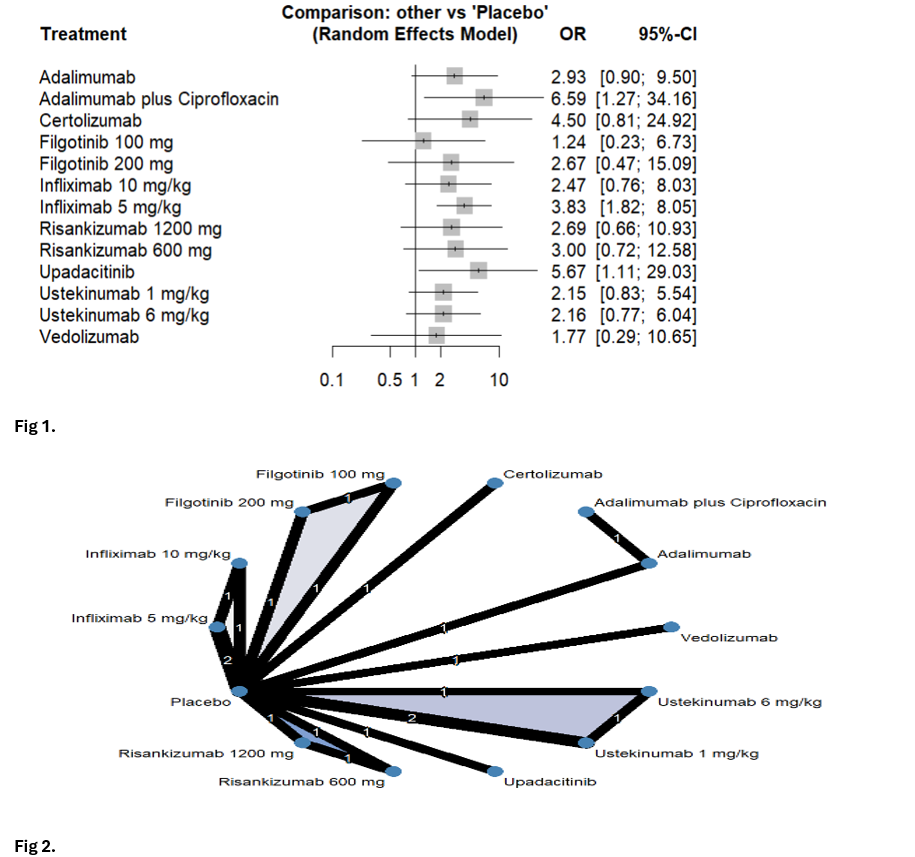
Figure: Fig 1. Forest plots of treatment comparisons for induction of fistula remission in fistulizing Crohn’s disease.; Fig 2. Network geometry of included treatments for the induction of remission and response in fistulizing Crohn’s disease.
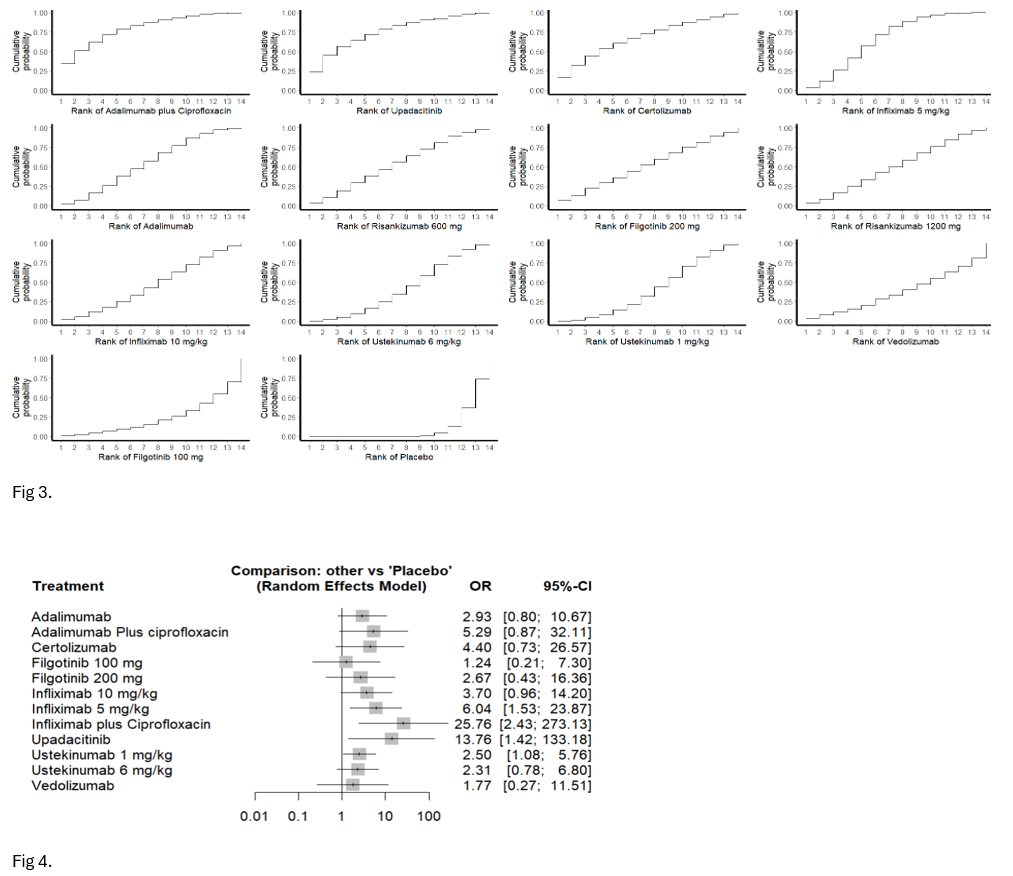
Figure: Fig 3. Treatment ranking based on P-scores for the induction of remission in fistulizing Crohn’s disease; Fig 4. Forest plots of treatment comparisons for induction of fistula response in fistulizing Crohn’s disease.
Disclosures:
Shahryar Khan indicated no relevant financial relationships.
Noor Ehsan indicated no relevant financial relationships.
Manav Nayeni indicated no relevant financial relationships.
Ryan Nazari indicated no relevant financial relationships.
Azzah Muhammad Hayat indicated no relevant financial relationships.
Ahmad Khan indicated no relevant financial relationships.
Hameed Ullah indicated no relevant financial relationships.
Asad Ur Rahman: Abbvie Inc – Speakers Bureau.
Shadi Hamdeh: Abbvie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau.
Shahryar Khan, MD1, Noor Ehsan, MD2, Manav Nayeni, BMSc3, Ryan Nazari, BS3, Azzah Muhammad Hayat, MD4, Ahmad Khan, 4, Hameed Ullah, MD5, Asad Ur Rahman, MD6, Shadi Hamdeh, MD, FACG1. P3196 - Efficacy of Biologics and Small Molecules for Induction of Response and Remission in Fistulizing Crohn's Disease: A Network Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Shahryar Khan, MD1, Noor Ehsan, MD2, Manav Nayeni, BMSc3, Ryan Nazari, BS3, Azzah Muhammad Hayat, MD4, Ahmad Khan, 4, Hameed Ullah, MD5, Asad Ur Rahman, MD6, Shadi Hamdeh, MD, FACG1
1University of Kansas Medical Center, Kansas City, KS; 2Gulab Devi Teaching Hospital, Lahore, Punjab, Pakistan; 3Kansas City University, Kansas City, MO; 4Khyber Medical University, Peshawar, North-West Frontier, Pakistan; 5St. Luke's Hospital, Chesterfield, MO; 6Cleveland Clinic Florida, Weston, FL
Introduction: Fistulizing Crohn’s disease (FCD) constitutes a complex and aggressive disease phenotype characterized by penetrating complications and substantial morbidity. The induction of clinical remission and fistula response remains a critical therapeutic objective; however, comparative efficacy data across the available advanced therapies are limited. This network meta-analysis (NMA) aimed to compare the efficacy of biologics and small molecules in inducing initial clinical remission and response in patients with FCD.
Methods: We conducted a systematic review and NMA of RCTs, and only studies reporting clinical outcomes with at least six months of follow-up were included. Fistula remission was defined as complete closure of all external draining fistulas and response as a ≥50% reduction in the number of draining fistulas from baseline without new fistula formation. A frequentist random-effects NMA was performed using the netmeta package in R to estimate the odds ratios and P-scores for treatment ranking.
Results: Thirteen RCTs were included to evaluate the efficacy of 14 advanced therapies. The predominant phenotype observed among the study participants was perianal FCD. For remission, Adalimumab plus Ciprofloxacin (OR 6.59; 95% CI 1.27-34.2), Infliximab 5 mg/kg (OR 3.83; 95% CI 1.82-8.05), and Upadacitinib (OR 5.67; 95% CI 1.11-29.03) significantly increased the odds of achieving complete fistula closure compared to placebo (Fig.1, Fig.2). Among the evaluated treatments, Adalimumab plus Ciprofloxacin ranked highest for induction of remission, followed by Upadacitinib (Fig.3). Regarding response, Infliximab plus Ciprofloxacin (OR 25.7; 95% CI 2.43-273), Infliximab 5 mg/kg (OR 6.04; 95% CI 1.53-23.8), Upadacitinib (OR 13.7; 95% CI 1.42-133.2), and Ustekinumab (OR 2.50; 95% CI 1.08-5.76) significantly improved clinical outcomes compared to placebo (Fig.4). Infliximab plus Ciprofloxacin ranked the highest for response, followed by Upadacitinib. Vedolizumab and Filgotinib did not demonstrate superiority over placebo in either remission or response.
Discussion: This analysis suggests that combination therapies, particularly those involving anti-TNF agents with antibiotics and novel agents, such as Upadacitinib, are associated with the most favorable induction of clinical remission and response in FCD. However, data on newer IL-23 inhibitors remain limited, underscoring the need for additional studies and head-to-head trials to better define their roles and guide optimal treatment selection.

Figure: Fig 1. Forest plots of treatment comparisons for induction of fistula remission in fistulizing Crohn’s disease.; Fig 2. Network geometry of included treatments for the induction of remission and response in fistulizing Crohn’s disease.

Figure: Fig 3. Treatment ranking based on P-scores for the induction of remission in fistulizing Crohn’s disease; Fig 4. Forest plots of treatment comparisons for induction of fistula response in fistulizing Crohn’s disease.
Disclosures:
Shahryar Khan indicated no relevant financial relationships.
Noor Ehsan indicated no relevant financial relationships.
Manav Nayeni indicated no relevant financial relationships.
Ryan Nazari indicated no relevant financial relationships.
Azzah Muhammad Hayat indicated no relevant financial relationships.
Ahmad Khan indicated no relevant financial relationships.
Hameed Ullah indicated no relevant financial relationships.
Asad Ur Rahman: Abbvie Inc – Speakers Bureau.
Shadi Hamdeh: Abbvie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau.
Shahryar Khan, MD1, Noor Ehsan, MD2, Manav Nayeni, BMSc3, Ryan Nazari, BS3, Azzah Muhammad Hayat, MD4, Ahmad Khan, 4, Hameed Ullah, MD5, Asad Ur Rahman, MD6, Shadi Hamdeh, MD, FACG1. P3196 - Efficacy of Biologics and Small Molecules for Induction of Response and Remission in Fistulizing Crohn's Disease: A Network Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

