Monday Poster Session
Category: IBD
P3193 - Intravenous and Subcutaneous Guselkumab Induction Therapy Are Both Efficacious in Crohn’s Disease Patients With High Baseline Disease Severity: Results at Week 12 From the Phase 3 GALAXI and GRAVITI Studies
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bruce E. Sands, MD, MS, FACG
Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
New York, NY
Presenting Author(s)
Award: ACG Presidential Poster Award
Bruce E. Sands, MD, MS, FACG1, Tadakazu Hisamatsu, MD, PhD2, Anita Afzali, MD, MPH, MHCM3, Nat A. Terry, MD, PhD4, Mobolaji Olurinde, MD, PhD4, Rian Van Rampelbergh, MD5, Jacqueline Yee, MS6, Wilbert van Duijnhoven, MSc5, Ailsa Hart, BA, BMBCh, PhD7, Silvio Danese, MD, PhD8, Remo Panaccione, MD9
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 3Department of Internal Medicine, Division of Digestive Diseases, University of Cincinnati College of Medicine, Cincinnati, OH, USA, Cincinnati, OH; 4Johnson & Johnson, Spring House, PA; 5Johnson & Johnson, Antwerp, Antwerpen, Belgium; 6Johnson & Johnson, Raritan, NJ; 7London North-West University Healthcare NHS Trust, London, England, United Kingdom; 8Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy; 9Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada
Introduction: The comparable efficacy and safety of both intravenous (IV) and subcutaneous (SC) induction with guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor, in participants (pts) with moderately to severely active Crohn’s disease (CD) has been established in the phase 3 GALAXI and GRAVITI studies. To determine whether pts with higher baseline disease severity respond differently to IV or SC induction, we compared the efficacy of GUS IV and SC induction in subgroups based on baseline disease characteristics.
Methods: Pts had moderately to severely active CD (CDAI 220-450 and SES-CD ≥6 or if ileal disease only, SES-CD ≥4), and a history of inadequate response/intolerance to oral corticosteroids, azathioprine/6-mercaptopurine/methotrexate, or biologics. GUS induction regimen was 200 mg IV q4w in GALAXI 2 & 3 (N=582) and 400 mg SC q4w in GRAVITI (N=230), while matching IV/SC PBO q4w (N=148 and N=117, respectively) was the comparator regimen. Clinical remission (CDAI< 150) and endoscopic response (≥50% improvement from baseline SES-CD) were evaluated at Week 12 for predefined subgroups based on baseline involved disease location (ie, ileum, colon, or both), CDAI (ie, ≤300 or > 300), SES-CD (ie, ≤12 or >12), fecal calprotectin (ie, ≤250 μ/g or >250 μ/g), and C-reactive protein (ie, >3 mg/L or ≤3 mg/L and >5 mg/L or ≤5 mg/L in GALAXI and GRAVITI, respectively). Abnormal C-reactive protein was defined as >3 mg/L and >5 mg/L for GALAXI and GRAVITI, respectively.
Results: GALAXI and GRAVITI had similar eligibility criteria, and baseline disease characteristics of the randomized study populations were also similar. Pts treated with IV or SC GUS induction achieved clinical remission at Week 12 in greater proportions than those who received PBO across all subgroups (Figure). Endoscopic response at Week 12 was also achieved by greater proportions of GUS- than PBO-induced pts across all subgroups with either IV or SC induction (Table).
Discussion: Both IV and SC induction with GUS were similarly effective in pts with moderately to severely active CD across predefined subgroups of disease location and baseline clinical, endoscopic, and inflammatory biomarker disease activity. The treatment effects were consistent in the subgroups following IV and SC induction, particularly in those that represent high disease severity.
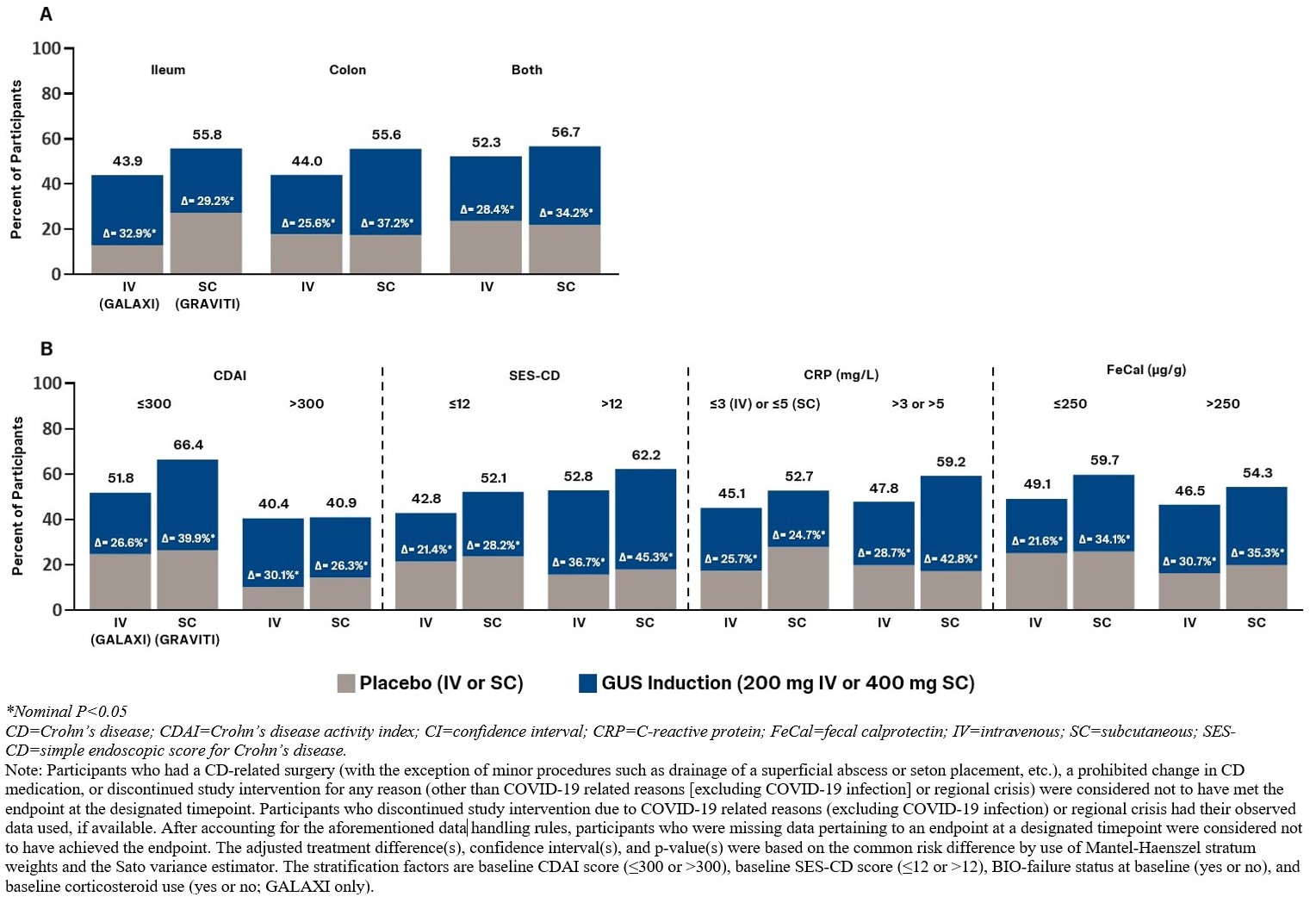
Figure: Figure. Clinical Remission at Week 12 in Subgroups by Disease Location (A), and Baseline Clinical, Endoscopic, and Inflammatory Biomarker Disease Activity (B)
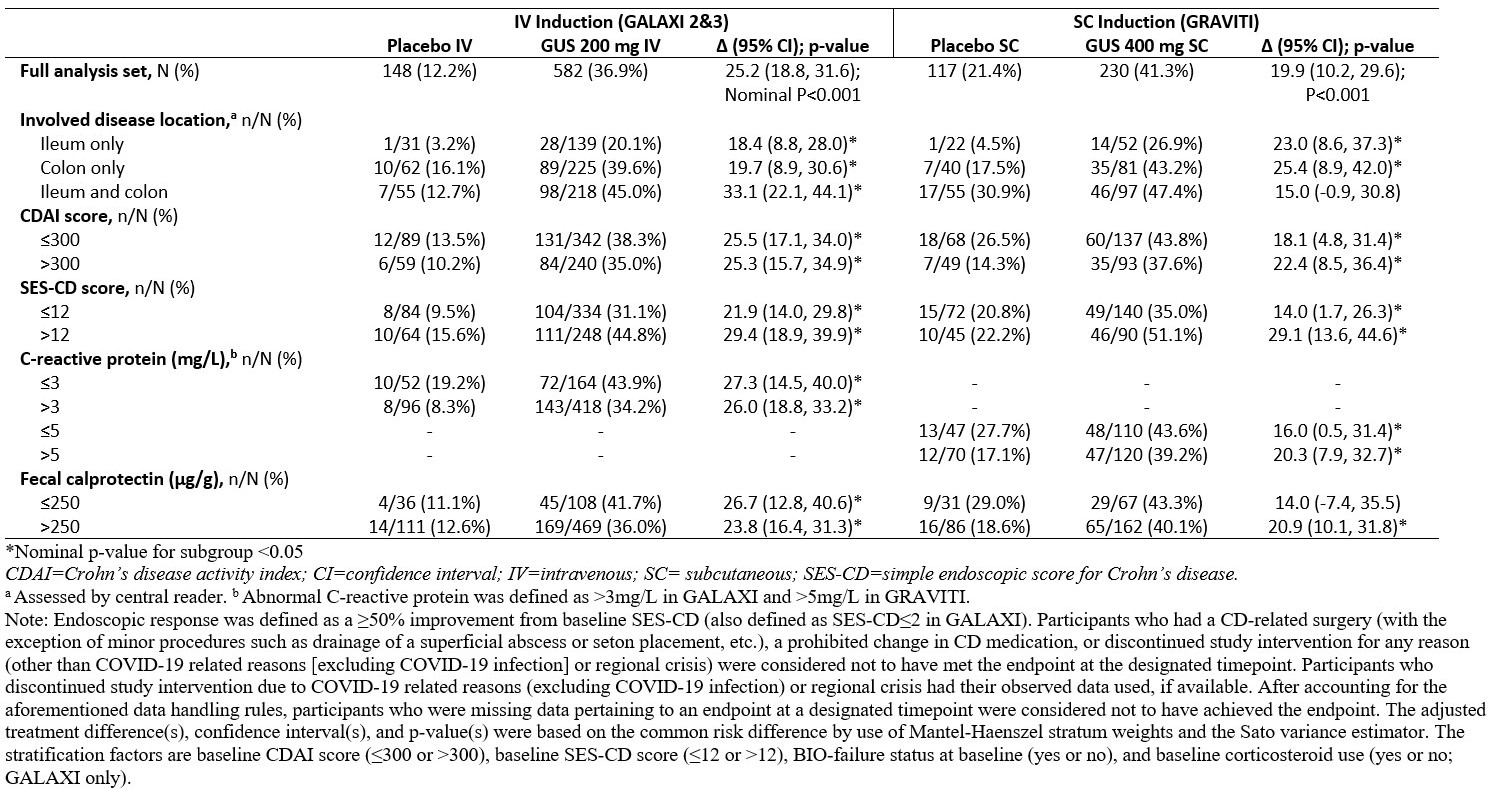
Figure: Table. Endoscopic Response at Week 12 by Baseline Disease Characteristics
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Anita Afzali: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Bristol Myers Squibb/Celgene – Consultant. DiaSorin – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Gilead – Consultant. IBD Horizons – Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker fees. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Scrubs & Heels Foundation – Advisory Committee/Board Member, Consultant, Co-Founder. Takeda – Advisory Committee/Board Member, Consultant, Speaker fees. TLL Pharmaceuticals – Consultant.
Nat Terry: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Mobolaji Olurinde: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rian Van Rampelbergh: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jacqueline Yee: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Wilbert van Duijnhoven: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ailsa Hart: AbbVie – Advisory Committee/Board Member, Lecturer. Bristol Myers Squibb – Advisory Committee/Board Member, Lecturer. Celltrion – Advisory Committee/Board Member, Lecturer. Falk – Advisory Committee/Board Member, Lecturer. Galapagos – Advisory Committee/Board Member, Lecturer. GSK – Advisory Committee/Board Member, Lecturer. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Lecturer. MSD – Advisory Committee/Board Member, Lecturer. Pfizer – Advisory Committee/Board Member, Lecturer. Takeda – Advisory Committee/Board Member, Lecturer.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Bruce E. Sands, MD, MS, FACG1, Tadakazu Hisamatsu, MD, PhD2, Anita Afzali, MD, MPH, MHCM3, Nat A. Terry, MD, PhD4, Mobolaji Olurinde, MD, PhD4, Rian Van Rampelbergh, MD5, Jacqueline Yee, MS6, Wilbert van Duijnhoven, MSc5, Ailsa Hart, BA, BMBCh, PhD7, Silvio Danese, MD, PhD8, Remo Panaccione, MD9. P3193 - Intravenous and Subcutaneous Guselkumab Induction Therapy Are Both Efficacious in Crohn’s Disease Patients With High Baseline Disease Severity: Results at Week 12 From the Phase 3 GALAXI and GRAVITI Studies, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Bruce E. Sands, MD, MS, FACG1, Tadakazu Hisamatsu, MD, PhD2, Anita Afzali, MD, MPH, MHCM3, Nat A. Terry, MD, PhD4, Mobolaji Olurinde, MD, PhD4, Rian Van Rampelbergh, MD5, Jacqueline Yee, MS6, Wilbert van Duijnhoven, MSc5, Ailsa Hart, BA, BMBCh, PhD7, Silvio Danese, MD, PhD8, Remo Panaccione, MD9
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 3Department of Internal Medicine, Division of Digestive Diseases, University of Cincinnati College of Medicine, Cincinnati, OH, USA, Cincinnati, OH; 4Johnson & Johnson, Spring House, PA; 5Johnson & Johnson, Antwerp, Antwerpen, Belgium; 6Johnson & Johnson, Raritan, NJ; 7London North-West University Healthcare NHS Trust, London, England, United Kingdom; 8Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy; 9Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada
Introduction: The comparable efficacy and safety of both intravenous (IV) and subcutaneous (SC) induction with guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor, in participants (pts) with moderately to severely active Crohn’s disease (CD) has been established in the phase 3 GALAXI and GRAVITI studies. To determine whether pts with higher baseline disease severity respond differently to IV or SC induction, we compared the efficacy of GUS IV and SC induction in subgroups based on baseline disease characteristics.
Methods: Pts had moderately to severely active CD (CDAI 220-450 and SES-CD ≥6 or if ileal disease only, SES-CD ≥4), and a history of inadequate response/intolerance to oral corticosteroids, azathioprine/6-mercaptopurine/methotrexate, or biologics. GUS induction regimen was 200 mg IV q4w in GALAXI 2 & 3 (N=582) and 400 mg SC q4w in GRAVITI (N=230), while matching IV/SC PBO q4w (N=148 and N=117, respectively) was the comparator regimen. Clinical remission (CDAI< 150) and endoscopic response (≥50% improvement from baseline SES-CD) were evaluated at Week 12 for predefined subgroups based on baseline involved disease location (ie, ileum, colon, or both), CDAI (ie, ≤300 or > 300), SES-CD (ie, ≤12 or >12), fecal calprotectin (ie, ≤250 μ/g or >250 μ/g), and C-reactive protein (ie, >3 mg/L or ≤3 mg/L and >5 mg/L or ≤5 mg/L in GALAXI and GRAVITI, respectively). Abnormal C-reactive protein was defined as >3 mg/L and >5 mg/L for GALAXI and GRAVITI, respectively.
Results: GALAXI and GRAVITI had similar eligibility criteria, and baseline disease characteristics of the randomized study populations were also similar. Pts treated with IV or SC GUS induction achieved clinical remission at Week 12 in greater proportions than those who received PBO across all subgroups (Figure). Endoscopic response at Week 12 was also achieved by greater proportions of GUS- than PBO-induced pts across all subgroups with either IV or SC induction (Table).
Discussion: Both IV and SC induction with GUS were similarly effective in pts with moderately to severely active CD across predefined subgroups of disease location and baseline clinical, endoscopic, and inflammatory biomarker disease activity. The treatment effects were consistent in the subgroups following IV and SC induction, particularly in those that represent high disease severity.

Figure: Figure. Clinical Remission at Week 12 in Subgroups by Disease Location (A), and Baseline Clinical, Endoscopic, and Inflammatory Biomarker Disease Activity (B)

Figure: Table. Endoscopic Response at Week 12 by Baseline Disease Characteristics
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Anita Afzali: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Bristol Myers Squibb/Celgene – Consultant. DiaSorin – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Gilead – Consultant. IBD Horizons – Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker fees. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Scrubs & Heels Foundation – Advisory Committee/Board Member, Consultant, Co-Founder. Takeda – Advisory Committee/Board Member, Consultant, Speaker fees. TLL Pharmaceuticals – Consultant.
Nat Terry: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Mobolaji Olurinde: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rian Van Rampelbergh: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jacqueline Yee: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Wilbert van Duijnhoven: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ailsa Hart: AbbVie – Advisory Committee/Board Member, Lecturer. Bristol Myers Squibb – Advisory Committee/Board Member, Lecturer. Celltrion – Advisory Committee/Board Member, Lecturer. Falk – Advisory Committee/Board Member, Lecturer. Galapagos – Advisory Committee/Board Member, Lecturer. GSK – Advisory Committee/Board Member, Lecturer. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Lecturer. MSD – Advisory Committee/Board Member, Lecturer. Pfizer – Advisory Committee/Board Member, Lecturer. Takeda – Advisory Committee/Board Member, Lecturer.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Bruce E. Sands, MD, MS, FACG1, Tadakazu Hisamatsu, MD, PhD2, Anita Afzali, MD, MPH, MHCM3, Nat A. Terry, MD, PhD4, Mobolaji Olurinde, MD, PhD4, Rian Van Rampelbergh, MD5, Jacqueline Yee, MS6, Wilbert van Duijnhoven, MSc5, Ailsa Hart, BA, BMBCh, PhD7, Silvio Danese, MD, PhD8, Remo Panaccione, MD9. P3193 - Intravenous and Subcutaneous Guselkumab Induction Therapy Are Both Efficacious in Crohn’s Disease Patients With High Baseline Disease Severity: Results at Week 12 From the Phase 3 GALAXI and GRAVITI Studies, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

