Monday Poster Session
Category: IBD
P3191 - Long-Term Efficacy of Etrasimod in Patients With Ulcerative Colitis: 2-Year Outcomes From the ELEVATE Open-Label Extension
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bruce E. Sands, MD, MS, FACG
Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
New York, NY
Presenting Author(s)
Award: ACG Presidential Poster Award
Bruce E. Sands, MD, MS, FACG1, Marla C. Dubinsky, MD2, Alessandro Armuzzi, MD, PhD3, Christopher Ma, MD, MPH4, Irina Blumenstein, MD5, Anita Afzali, MD, MPH, MHCM6, Patryk Smolinski, MD7, Michelle Segovia, PharmD8, Diogo Branquinho, MD, MSc, FEBGH8, John C. Woolcott, PhD9, Martina Goetsch, MD10, Krisztina Lazin, MD10, Micheal Keating, PharmD8, Chuanbo Zang, PhD9, David T. Rubin, MD11, Silvio Danese, MD, PhD12
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 3IBD Unit, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy, Milan, Lombardia, Italy; 4University of Calgary, Calgary, AB, Canada; 5Goethe University Frankfurt, Frankfurt University Hospital, Medical Clinic 1, 60596 Frankfurt am Main, Germany, Frankfurt am Main, Hessen, Germany; 6Department of Internal Medicine, Division of Digestive Diseases, University of Cincinnati College of Medicine, Cincinnati, OH, USA, Cincinnati, OH; 7EuroMediCare Szpital Specjalistyczny z Przychodnia, Wrocław, Poland, Wrocław, Dolnoslaskie, Poland; 8Pfizer Inc, New York, NY, USA, New York, NY; 9Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 10Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 11University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 12Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). The efficacy and safety of etrasimod were demonstrated in the phase 3 ELEVATE UC clinical program.1 This is the first report of the long-term efficacy of etrasimod 2 mg QD from the ongoing phase 3 open-label extension (OLE) study, ELEVATE UC OLE (NCT03950232). Pooled safety data up to 4 years from the global clinical program have been previously reported.2
Methods: Patients (pts) entered the OLE from qualifying UC parent studies if they completed the parent studies or experienced disease worsening during the maintenance period of the parent study (Figure). Efficacy endpoints included blinded centrally-read endoscopy, performed at Week (Wk) 52 and Wk 104 of the OLE. Endpoints were assessed among pts who entered the OLE as clinical responders to etrasimod 2 mg QD in the parent studies (data cut-off: June 20, 2024). Data are reported using observed cases with corresponding modified nonresponder imputation (mNRI) rates.
Results: A total of 296 pts were included, with a mean (standard deviation) etrasimod exposure of 120.0 (50.3; range: 3.0–218.3) wks. Most pts (n = 252; 85.1%) received ≥ 52 wks and 207 (69.9%) received ≥ 104 wks of open-label treatment. For this analysis, treatment was ongoing in 186 (62.8%) pts, while 109 (36.8%) had discontinued; reasons for discontinuation included pt withdrawal (n = 38; 12.8%), adverse events (n = 38; 12.8%), and lack of efficacy (n = 20; 6.8%). Efficacy outcomes are summarized in the Table. In the OLE, clinical response was observed in 89.3% (mNRI: 74.4%) and 87.6% (61.8%) of pts at Wks 52 and 104, respectively. At Wk 52 and Wk 104, clinical remission rates were 56.6% (47.1%) and 55.2% (38.9%), respectively. Endoscopic improvement was observed in ~ 65% of pts at both timepoints (Wk 52: 53.2%; Wk 104: 43.9%). Symptomatic response and symptomatic remission were observed in ~ 95% (Wk 52: 78.5%; Wk 104: 66.0%) and ~ 80% (Wk 52: 66.9%; Wk 104: 54.7%) of pts, respectively, through Wk 104.
Discussion: Etrasimod efficacy was maintained through an additional 2 years of continuous treatment across clinical, endoscopic, and symptomatic outcomes, supporting long-term durable efficacy with etrasimod treatment. This study is ongoing with future expected readouts.
References:
1. Sandborn WJ et al. Lancet 2023; 401: 1159–1171.
2. Vermeire S et al. J Crohns Colitis 2025; 19: i119–i121.
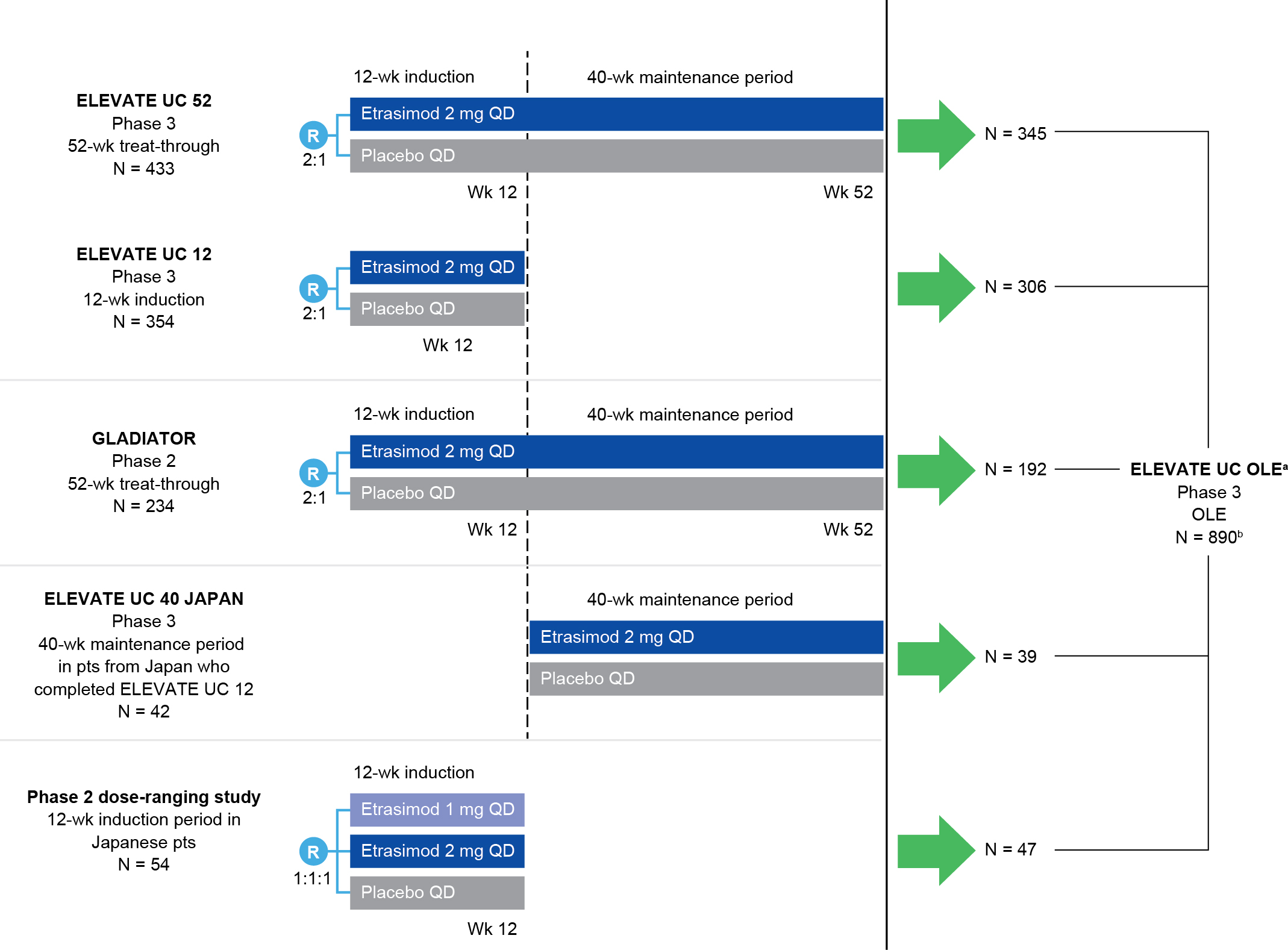
Figure: Figure. ELEVATE UC OLE parent studies.
[a]Pts were eligible to enter ELEVATE UC OLE if they completed the parent study or they experienced disease worsening during the 40-wk treatment period of the parent study, provided their ES was ≥ 2 and they met one of the following entry criteria: RBS ≥ 2 at two timepoints at least 7 days and no more than 14 days apart; RBS + SFS ≥ 4 at two timepoints at least 7 days and no more than 14 days apart; RBS ≥ 2 or RBS + SFS ≥ 4 (in any order) at two timepoints at least 7 days and no more than 14 days apart.
[b]Full analysis set (baseline MMS 4–9). For pts who completed ELEVATE UC 12 and entered ELEVATE UC 40 JAPAN, disposition was counted separately in both studies; therefore, N = 890 includes pts from ELEVATE UC 52, ELEVATE UC 12, GLADIATOR, and the phase 2 dose-ranging study.
ES, endoscopic subscore; N, number of pts in cohort; OLE, open-label extension; pt, patient; QD, once daily; R, randomization; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis; wk, week.
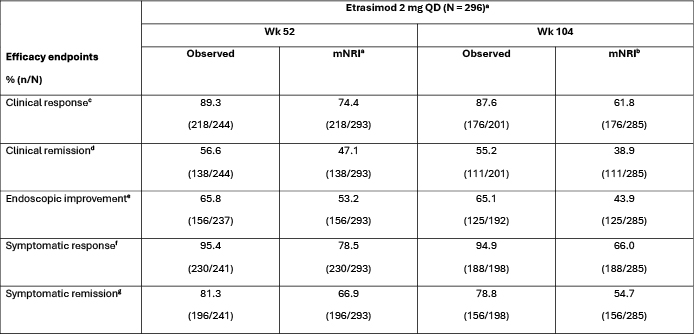
Figure: Table. Efficacy at Wk 52 and Wk 104 of ELEVATE UC OLE among etrasimod responders in parent studies.
Eligible pts were those who received etrasimod 2 mg QD in the parent studies (ELEVATE UC 52, ELEVATE UC 12, ELEVATE UC 40 JAPAN, GLADIATOR, and a phase 2 dose-ranging study with Japanese pts) and who were in clinical response at the last visit in the parent study prior to entering the ELEVATE UC OLE. Baseline in applicable endpoint definitions refers to the day of first dose in the parent study from which patients entered the OLE.
[a]At the time of the analysis, treatment was ongoing in 186 (62.8%) pts, while 109 (36.8%) pts had discontinued; 1 (0.3%) pt had completed treatment at the time of the analysis.
[b]mNRI includes in the denominator those pts who had reached the corresponding time point and those who withdrew before the time point but would have reached the time point if they had stayed. Pts with missing data were treated as nonresponders.
[c]Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1.
[d]Clinical remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[e]Endoscopic improvement was defined as ES ≤ 1.
[f]Symptomatic response was defined as a ≥ 30% decrease from baseline in composite RBS and SFS.
[g]Symptomatic remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline) and RBS = 0.
ES, endoscopic subscore; MMS, modified Mayo score; mNRI, modified nonresponder imputation; N, number of pts in cohort; n, number of pts; OLE, open-label extension; pt, patient; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis; wk, week.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Alessandro Armuzzi: AbbVie – Consultant, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AG Pharma – Speakers Bureau. Alfa Sigma – Consultant, Speakers Bureau. AstraZeneca – Consultant. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Enthera Pharmaceuticals – Consultant. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead Sciences – Consultant, Speakers Bureau. Giuliani – Consultant. Janssen – Consultant, Speakers Bureau. Lionhealth – Consultant, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Nestle – Consultant. Novartis – Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant. Roche – Consultant, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Sanofi – Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Teva Pharmaceuticals – Consultant, Speakers Bureau. Tillotts – Consultant.
Christopher Ma: Abbvie – Consultant, Grant/Research Support, speaker's fee. Alimentiv – Consultant, speaker's fee. Amgen – Consultant, speaker's fee. AVIR Pharma Inc. – Consultant, speaker's fee. Bristol Myers Squibb – Consultant, speaker's fee. Celltrion – Consultant. Eli Lilly – Consultant, Grant/Research Support, speaker's fee. Ferring – Consultant, Grant/Research Support, speaker's fee. Forte Biosciences – Consultant. Fresenius Kabi – Consultant, speaker's fee. Gilead – Consultant. Janssen – Consultant, speaker's fee. McKesson – Consultant. Merck – Speaker's fee. Mirador Therapeutics – Consultant. Mylan – Consultant. Organon – speaker's fee. Pendopharm – Consultant, speaker's fee. Pfizer – Consultant, Grant/Research Support, speaker's fee. Prometheus Biosciences Inc. – Consultant. Roche – Consultant. Sanofi – Consultant, speaker's fee. Springer Publishing – Royalties. Takeda – Consultant, speaker's fee. Tillotts Pharma – Consultant, speaker's fee.
Irina Blumenstein: AbbVie – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Celgene/BMS – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Falk Foundation – Consultant, Speakers Bureau. Fresenius Kabi – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. GmbH – Consultant, Speakers Bureau. Janssen-Cilag – Consultant, Speakers Bureau. Lilly – Consultant, Speakers Bureau. Merck – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Pharmacosmos – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau.
Anita Afzali: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Bristol Myers Squibb/Celgene – Consultant. DiaSorin – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Gilead – Consultant. IBD Horizons – Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker fees. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Scrubs & Heels Foundation – Advisory Committee/Board Member, Consultant, Co-Founder. Takeda – Advisory Committee/Board Member, Consultant, Speaker fees. TLL Pharmaceuticals – Consultant.
Patryk Smolinski: Polpharma – Speakers Bureau. PRO.MED.CS Praha a.s – Consultant, Speakers Bureau. URGO – Consultant.
Michelle Segovia: Pfizer Inc – Employee.
Diogo Branquinho: Pfizer Inc – Employee, Stock Options.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Micheal Keating: Pfizer Inc – Employee, Stock Options.
Chuanbo Zang: Pfizer Inc – Employee, Stock Options.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Bruce E. Sands, MD, MS, FACG1, Marla C. Dubinsky, MD2, Alessandro Armuzzi, MD, PhD3, Christopher Ma, MD, MPH4, Irina Blumenstein, MD5, Anita Afzali, MD, MPH, MHCM6, Patryk Smolinski, MD7, Michelle Segovia, PharmD8, Diogo Branquinho, MD, MSc, FEBGH8, John C. Woolcott, PhD9, Martina Goetsch, MD10, Krisztina Lazin, MD10, Micheal Keating, PharmD8, Chuanbo Zang, PhD9, David T. Rubin, MD11, Silvio Danese, MD, PhD12. P3191 - Long-Term Efficacy of Etrasimod in Patients With Ulcerative Colitis: 2-Year Outcomes From the ELEVATE Open-Label Extension, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Bruce E. Sands, MD, MS, FACG1, Marla C. Dubinsky, MD2, Alessandro Armuzzi, MD, PhD3, Christopher Ma, MD, MPH4, Irina Blumenstein, MD5, Anita Afzali, MD, MPH, MHCM6, Patryk Smolinski, MD7, Michelle Segovia, PharmD8, Diogo Branquinho, MD, MSc, FEBGH8, John C. Woolcott, PhD9, Martina Goetsch, MD10, Krisztina Lazin, MD10, Micheal Keating, PharmD8, Chuanbo Zang, PhD9, David T. Rubin, MD11, Silvio Danese, MD, PhD12
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 3IBD Unit, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy, Milan, Lombardia, Italy; 4University of Calgary, Calgary, AB, Canada; 5Goethe University Frankfurt, Frankfurt University Hospital, Medical Clinic 1, 60596 Frankfurt am Main, Germany, Frankfurt am Main, Hessen, Germany; 6Department of Internal Medicine, Division of Digestive Diseases, University of Cincinnati College of Medicine, Cincinnati, OH, USA, Cincinnati, OH; 7EuroMediCare Szpital Specjalistyczny z Przychodnia, Wrocław, Poland, Wrocław, Dolnoslaskie, Poland; 8Pfizer Inc, New York, NY, USA, New York, NY; 9Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 10Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 11University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 12Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). The efficacy and safety of etrasimod were demonstrated in the phase 3 ELEVATE UC clinical program.1 This is the first report of the long-term efficacy of etrasimod 2 mg QD from the ongoing phase 3 open-label extension (OLE) study, ELEVATE UC OLE (NCT03950232). Pooled safety data up to 4 years from the global clinical program have been previously reported.2
Methods: Patients (pts) entered the OLE from qualifying UC parent studies if they completed the parent studies or experienced disease worsening during the maintenance period of the parent study (Figure). Efficacy endpoints included blinded centrally-read endoscopy, performed at Week (Wk) 52 and Wk 104 of the OLE. Endpoints were assessed among pts who entered the OLE as clinical responders to etrasimod 2 mg QD in the parent studies (data cut-off: June 20, 2024). Data are reported using observed cases with corresponding modified nonresponder imputation (mNRI) rates.
Results: A total of 296 pts were included, with a mean (standard deviation) etrasimod exposure of 120.0 (50.3; range: 3.0–218.3) wks. Most pts (n = 252; 85.1%) received ≥ 52 wks and 207 (69.9%) received ≥ 104 wks of open-label treatment. For this analysis, treatment was ongoing in 186 (62.8%) pts, while 109 (36.8%) had discontinued; reasons for discontinuation included pt withdrawal (n = 38; 12.8%), adverse events (n = 38; 12.8%), and lack of efficacy (n = 20; 6.8%). Efficacy outcomes are summarized in the Table. In the OLE, clinical response was observed in 89.3% (mNRI: 74.4%) and 87.6% (61.8%) of pts at Wks 52 and 104, respectively. At Wk 52 and Wk 104, clinical remission rates were 56.6% (47.1%) and 55.2% (38.9%), respectively. Endoscopic improvement was observed in ~ 65% of pts at both timepoints (Wk 52: 53.2%; Wk 104: 43.9%). Symptomatic response and symptomatic remission were observed in ~ 95% (Wk 52: 78.5%; Wk 104: 66.0%) and ~ 80% (Wk 52: 66.9%; Wk 104: 54.7%) of pts, respectively, through Wk 104.
Discussion: Etrasimod efficacy was maintained through an additional 2 years of continuous treatment across clinical, endoscopic, and symptomatic outcomes, supporting long-term durable efficacy with etrasimod treatment. This study is ongoing with future expected readouts.
References:
1. Sandborn WJ et al. Lancet 2023; 401: 1159–1171.
2. Vermeire S et al. J Crohns Colitis 2025; 19: i119–i121.

Figure: Figure. ELEVATE UC OLE parent studies.
[a]Pts were eligible to enter ELEVATE UC OLE if they completed the parent study or they experienced disease worsening during the 40-wk treatment period of the parent study, provided their ES was ≥ 2 and they met one of the following entry criteria: RBS ≥ 2 at two timepoints at least 7 days and no more than 14 days apart; RBS + SFS ≥ 4 at two timepoints at least 7 days and no more than 14 days apart; RBS ≥ 2 or RBS + SFS ≥ 4 (in any order) at two timepoints at least 7 days and no more than 14 days apart.
[b]Full analysis set (baseline MMS 4–9). For pts who completed ELEVATE UC 12 and entered ELEVATE UC 40 JAPAN, disposition was counted separately in both studies; therefore, N = 890 includes pts from ELEVATE UC 52, ELEVATE UC 12, GLADIATOR, and the phase 2 dose-ranging study.
ES, endoscopic subscore; N, number of pts in cohort; OLE, open-label extension; pt, patient; QD, once daily; R, randomization; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis; wk, week.

Figure: Table. Efficacy at Wk 52 and Wk 104 of ELEVATE UC OLE among etrasimod responders in parent studies.
Eligible pts were those who received etrasimod 2 mg QD in the parent studies (ELEVATE UC 52, ELEVATE UC 12, ELEVATE UC 40 JAPAN, GLADIATOR, and a phase 2 dose-ranging study with Japanese pts) and who were in clinical response at the last visit in the parent study prior to entering the ELEVATE UC OLE. Baseline in applicable endpoint definitions refers to the day of first dose in the parent study from which patients entered the OLE.
[a]At the time of the analysis, treatment was ongoing in 186 (62.8%) pts, while 109 (36.8%) pts had discontinued; 1 (0.3%) pt had completed treatment at the time of the analysis.
[b]mNRI includes in the denominator those pts who had reached the corresponding time point and those who withdrew before the time point but would have reached the time point if they had stayed. Pts with missing data were treated as nonresponders.
[c]Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1.
[d]Clinical remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[e]Endoscopic improvement was defined as ES ≤ 1.
[f]Symptomatic response was defined as a ≥ 30% decrease from baseline in composite RBS and SFS.
[g]Symptomatic remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline) and RBS = 0.
ES, endoscopic subscore; MMS, modified Mayo score; mNRI, modified nonresponder imputation; N, number of pts in cohort; n, number of pts; OLE, open-label extension; pt, patient; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis; wk, week.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Alessandro Armuzzi: AbbVie – Consultant, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AG Pharma – Speakers Bureau. Alfa Sigma – Consultant, Speakers Bureau. AstraZeneca – Consultant. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Enthera Pharmaceuticals – Consultant. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead Sciences – Consultant, Speakers Bureau. Giuliani – Consultant. Janssen – Consultant, Speakers Bureau. Lionhealth – Consultant, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Nestle – Consultant. Novartis – Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant. Roche – Consultant, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Sanofi – Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Teva Pharmaceuticals – Consultant, Speakers Bureau. Tillotts – Consultant.
Christopher Ma: Abbvie – Consultant, Grant/Research Support, speaker's fee. Alimentiv – Consultant, speaker's fee. Amgen – Consultant, speaker's fee. AVIR Pharma Inc. – Consultant, speaker's fee. Bristol Myers Squibb – Consultant, speaker's fee. Celltrion – Consultant. Eli Lilly – Consultant, Grant/Research Support, speaker's fee. Ferring – Consultant, Grant/Research Support, speaker's fee. Forte Biosciences – Consultant. Fresenius Kabi – Consultant, speaker's fee. Gilead – Consultant. Janssen – Consultant, speaker's fee. McKesson – Consultant. Merck – Speaker's fee. Mirador Therapeutics – Consultant. Mylan – Consultant. Organon – speaker's fee. Pendopharm – Consultant, speaker's fee. Pfizer – Consultant, Grant/Research Support, speaker's fee. Prometheus Biosciences Inc. – Consultant. Roche – Consultant. Sanofi – Consultant, speaker's fee. Springer Publishing – Royalties. Takeda – Consultant, speaker's fee. Tillotts Pharma – Consultant, speaker's fee.
Irina Blumenstein: AbbVie – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Celgene/BMS – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Falk Foundation – Consultant, Speakers Bureau. Fresenius Kabi – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. GmbH – Consultant, Speakers Bureau. Janssen-Cilag – Consultant, Speakers Bureau. Lilly – Consultant, Speakers Bureau. Merck – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Pharmacosmos – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau.
Anita Afzali: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Bristol Myers Squibb/Celgene – Consultant. DiaSorin – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Gilead – Consultant. IBD Horizons – Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker fees. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Scrubs & Heels Foundation – Advisory Committee/Board Member, Consultant, Co-Founder. Takeda – Advisory Committee/Board Member, Consultant, Speaker fees. TLL Pharmaceuticals – Consultant.
Patryk Smolinski: Polpharma – Speakers Bureau. PRO.MED.CS Praha a.s – Consultant, Speakers Bureau. URGO – Consultant.
Michelle Segovia: Pfizer Inc – Employee.
Diogo Branquinho: Pfizer Inc – Employee, Stock Options.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Micheal Keating: Pfizer Inc – Employee, Stock Options.
Chuanbo Zang: Pfizer Inc – Employee, Stock Options.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Bruce E. Sands, MD, MS, FACG1, Marla C. Dubinsky, MD2, Alessandro Armuzzi, MD, PhD3, Christopher Ma, MD, MPH4, Irina Blumenstein, MD5, Anita Afzali, MD, MPH, MHCM6, Patryk Smolinski, MD7, Michelle Segovia, PharmD8, Diogo Branquinho, MD, MSc, FEBGH8, John C. Woolcott, PhD9, Martina Goetsch, MD10, Krisztina Lazin, MD10, Micheal Keating, PharmD8, Chuanbo Zang, PhD9, David T. Rubin, MD11, Silvio Danese, MD, PhD12. P3191 - Long-Term Efficacy of Etrasimod in Patients With Ulcerative Colitis: 2-Year Outcomes From the ELEVATE Open-Label Extension, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

