Monday Poster Session
Category: IBD
P3189 - Maintenance of Endoscopic and Histologic Efficacy With Guselkumab for Ulcerative Colitis at Week 92 of the QUASAR Long-Term Extension Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bruce E. Sands, MD, MS, FACG
Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
New York, NY
Presenting Author(s)
Award: ACG Presidential Poster Award
Tadakazu Hisamatsu, MD, PhD1, Julián Panés, MD2, Fernando Magro, MD, PhD3, Gary R.. Lichtenstein, MD, FACG4, Jessica R.. Allegretti, MD, MPH5, Brian Bressler, MD, MS6, Waqqas Afif, MD7, Mark A. Samaan, MD, MBBS8, Byong Duk Ye, MD, PhD9, Shadi Yarandi, MD10, Matthew Germinaro, MD10, Nicole Shipitofsky, PharmD10, Dwiti Pandya, MS10, Ye Miao, MS10, Hongyan Zhang, 10, Axel Dignass, MD, PhD11, Bruce E. Sands, MD, MS, FACG12
1Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 2Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 3University of Porto, Porto, Porto, Portugal; 4University of Pennsylvania, Philadelphia, PA; 5Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 6University of British Columbia, Vancouver, BC, Canada; 7Division of Gastroenterology, McGill University Health Care, Montreal, PQ, Canada; 8Inflammatory Bowel Disease Unit, Guy’s and St Thomas’ NHS Foundation Trust, London, England, United Kingdom; 9University of Ulsan College of Medicine, Asan Medical Center, Seoul, Seoul-t'ukpyolsi, Republic of Korea; 10Johnson & Johnson, Spring House, PA; 11Agaplesion Markus Hospital, Frankfurt, Hessen, Germany; 12Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY
Introduction: Guselkumab (GUS) is a dual-acting IL-23p19 subunit inhibitor that potently neutralizes IL-23 and binds to CD64, a receptor on cells that produce IL-23. In the QUASAR studies, GUS intravenous (IV) induction and subcutaneous (SC) maintenance demonstrated symptomatic, endoscopic, and histologic efficacy through 2 years.1,2 Here, we describe maintenance of endoscopic and histologic efficacy in the long-term extension (LTE).
Methods: At maintenance week (W) 0, GUS IV induction responders were randomized 1:1:1 to GUS 100 mg SC every 8 weeks (q8w), GUS 200 mg SC q4w or placebo (GUS withdrawal). Participants (pts) completing the W44 visit could enter the LTE and continue the treatment regimen they were receiving at W44. Placebo pts discontinued after study unblinding. Efficacy data for randomized pts who continued to receive their GUS regimen assigned at W0 in the LTE were analyzed using 2 methods: 1) nonresponder imputation (NRI) accounting for pts with treatment failure or missing data, and 2) “as observed.”
Results: Overall, 303 randomized pts (155 for 100 mg q8w and 148 for 200 mg q4w) continued their GUS treatment assigned at W0 in the LTE. Of pts who achieved endoscopic improvement at W44, 87.2% of GUS 100 mg q8w pts and 80.2% GUS 200 mg q4w pts maintained endoscopic improvement through W92 (NRI analysis; Figure 1). Of pts who achieved histologic improvement at W44, 77.9% and 75.2%, respectively, maintained histologic improvement at W92. Of pts who achieved histo-endoscopic mucosal improvement (HEMI) at W44, 73.5% and 75.5%, respectively, maintained HEMI through W92. Of pts who were in histologic remission at W44, 71.2% maintained histologic remission at W92 in both GUS groups. Results of the as-observed analysis were consistent with the NRI analysis; the dropout rate was low among GUS-treated pts in the LTE. Proportions of pts who maintained endoscopic and histologic outcomes from W0 to W92 are presented in Figure 2.
Discussion: High rates of long-term sustained endoscopic and histologic endpoints were observed at W92 of the LTE with both GUS dose regimens.
References: 1. Rubin DT, et al. Lancet. 2025;405:33-49; 2. Lichtenstein GR, et al. Presented at DDW 2025; May 4–6, 2025; San Diego, CA.
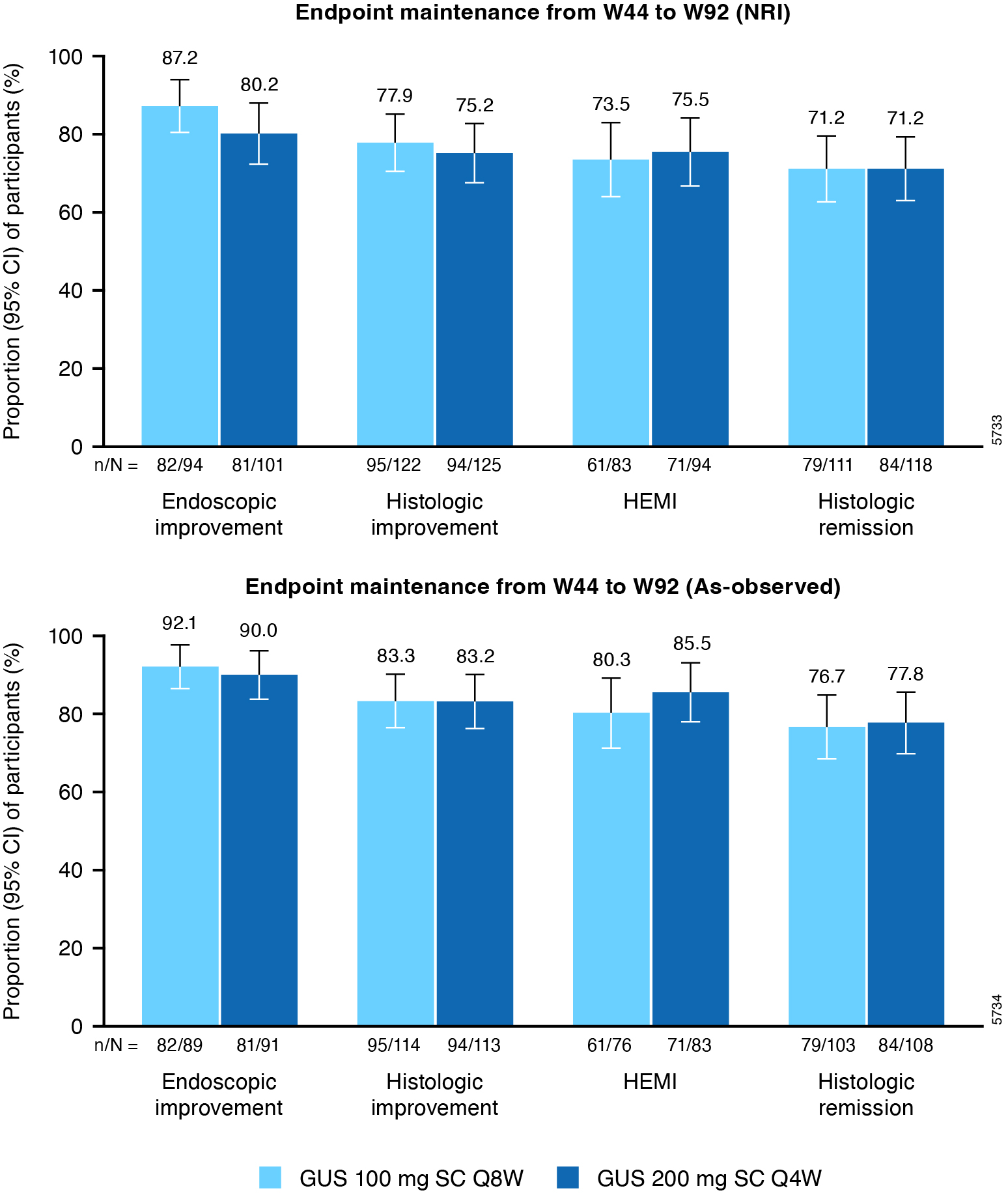
Figure: Figure 1. Maintenance of endoscopic and histological efficacy with GUS from W44 to W92 in the QUASAR LTE study based on NRI and as-observed analysis.
Includes pts with modified Mayo score of 5-9 at induction baseline who achieved clinical response to GUS IV induction and were randomized to receive GUS maintenance treatment and did not experience a dose adjustment from W8 through W32.
Proportions of pts reflect pts achieving the endpoint at W92 among those who achieved the corresponding endpoint at W44.
Endoscopic improvement is defined as an endoscopic subscore of 0 or 1. Histologic improvement is defined as neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system. HEMI is defined as achieving a combination of histologic improvement and endoscopic improvement. Histologic remission is defined as the absence of neutrophils from the mucosa (both lamina propria and epithelium), no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system (equivalent to a Robarts Histopathology Index ≤2b, with subscores of 0 for lamina propria neutrophils and neutrophils in the epithelium and without ulcers or erosion).
CI=Confidence interval; GUS=Guselkumab; HEMI=histologic-endoscopic mucosal improvement; IV=Intravenous; LTE=Long-term extension; NRI=Nonresponder imputation; pts=Participants; q4w=Every 4 weeks; q8w=Every 8 weeks; SC=Subcutaneous; W=Week.
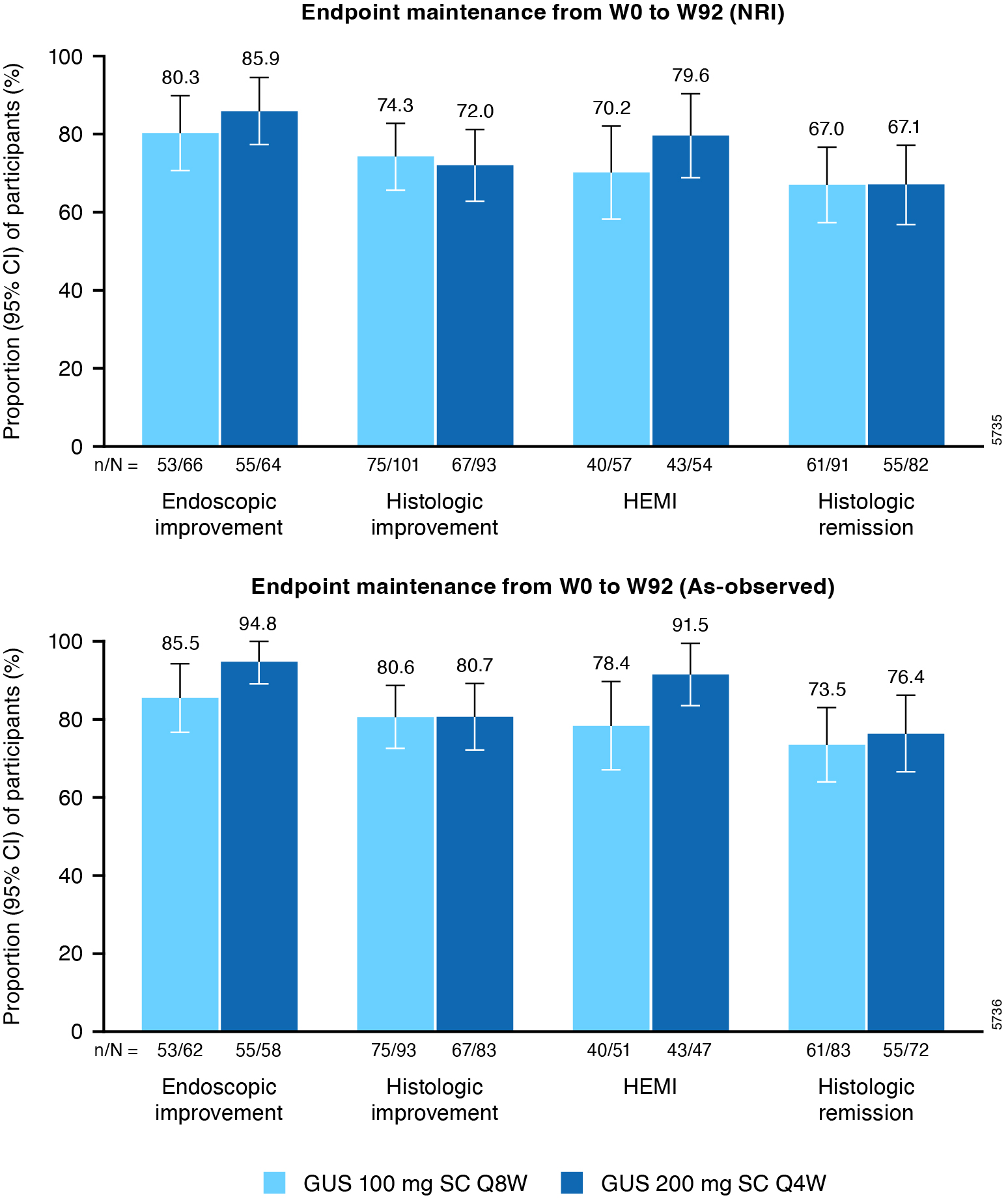
Figure: Figure 2. Maintenance of endoscopic and histological efficacy with GUS from W0 to W92 in the QUASAR LTE study based on NRI and as-observed analysis.
Includes pts with modified Mayo score of 5-9 at induction baseline who achieved clinical response to GUS IV induction and were randomized to receive GUS maintenance treatment and did not experience a dose adjustment from W8 through W32.
Proportions of pts reflect pts achieving the endpoint at W92 among those who achieved the corresponding endpoint at W0.
Endoscopic improvement is defined as an endoscopic subscore of 0 or 1. Histologic improvement is defined as neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system. HEMI is defined as achieving a combination of histologic improvement and endoscopic improvement. Histologic remission is defined as the absence of neutrophils from the mucosa (both lamina propria and epithelium), no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system (equivalent to a Robarts Histopathology Index ≤2b, with subscores of 0 for lamina propria neutrophils and neutrophils in the epithelium and without ulcers or erosion).
CI=Confidence interval; GUS=Guselkumab; HEMI=histologic-endoscopic mucosal improvement; IV=Intravenous; LTE=Long-term extension; NRI=Nonresponder imputation; pts=Participants; q4w=Every 4 weeks; q8w=Every 8 weeks; SC=Subcutaneous; W=Week.
Disclosures:
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Julián Panés: AbbVie – Consultant. Alimentiv – Consultant, Data safety monitoring board. Athos – Consultant. Atomwise – Consultant. Boehringer Ingelheim – Consultant. Celsius – Consultant. Ferring – Consultant. Galapagos – Consultant. Genentech/Roche – Consultant. GlaxoSmithKline – Consultant. Johnson & Johnson – Consultant. Mirum – Consultant, Data safety monitoring board. Nimbus – Consultant. Pfizer – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist – Consultant. Revolo – Consultant. Sanofi – Consultant, Data safety monitoring board. Sorriso – Consultant, Data safety monitoring board. Surrozen – Consultant, Data safety monitoring board. Takeda – Consultant. Wasserman – Consultant.
Fernando Magro: AbbVie – Speakers Bureau. Arena Pharmaceuticals – Speakers Bureau. Biogen – Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring Pharmaceuticals – Speakers Bureau. Hospira – Speakers Bureau. Janssen – Speakers Bureau. Laboratórios Vitoria – Speakers Bureau. Merck Sharp & Dohme – Speakers Bureau. Pfizer – Speakers Bureau. Sandoz – Speakers Bureau. Takeda – Speakers Bureau. UCB Pharma – Speakers Bureau. Vifor Pharma – Speakers Bureau.
Gary Lichtenstein: Abbvie – Consultant. American College of Gastroenterology – honorarium as associate editor of American Journal of Gastroenterology. Celgene – Consultant, Grant/Research Support. CellCeutrix – Consultant. Clinical Advances in Gastroenterology – Honorarium - Associate Editor. Eli Lilly – Consultant, DSMB. Ferring – Consultant. Gastroenterology and Hepatology [Gastro-Hep Communications] – Honorarium - Editor. Gilead – Consultant. Johnson & Johnson – Consultant. Luitpold/American Regent – Consultant, Honorarium - CME programs. McMahon Publishing – Honorarium - Author. Merck – Consultant, Honorarium - CME programs. Orthobiotech – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Romark – Consultant, Honorarium - CME programs. Salix/Valeant – Consultant, Grant/Research Support. Shire – Consultant, Grant/Research Support. SLACK – Book royalty. Springer – Honorarium - Editor. Takeda – Consultant, Funding to institution [IBD fellow education]. UCB – Consultant, Grant/Research Support. Up-To-Date – Honorarium - Author. Vindico – CME. Virgo – Consultant, Stock Options.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Brian Bressler: Abbvie – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Amgen – Advisor or Review Panel Member, Grant/Research Support. AMT – Advisor or Review Panel Member. Bausch Health – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Celgene – Advisor or Review Panel Member. Celltrion – Advisor or Review Panel Member. Eupraxia Fresenius Kabi – Advisor or Review Panel Member. Ferring – Advisor or Review Panel Member, Speakers Bureau. Genentech/Roche – Advisor or Review Panel Member, Grant/Research Support. Gilead – Advisor or Review Panel Member. GlaxoSmithKline – Grant/Research Support. Iterative Scopes – Advisor or Review Panel Member. Jamp – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Mylan – Advisor or Review Panel Member. Novartis – Advisor or Review Panel Member, Speakers Bureau. Organaon – Advisor or Review Panel Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Takeda – Advisor or Review Panel Member, Speakers Bureau. Viatris – Advisor or Review Panel Member.
Waqqas Afif: Abbvie – Speaker, advisory board member and/or clinical investigator. Amgen – Speaker, advisory board member and/or clinical investigator. Eli Lilly – Speaker, advisory board member and/or clinical investigator. Johnson & Johnson – Speaker, advisory board member and/or clinical investigator. Merck – Speaker, advisory board member and/or clinical investigator. Novartis – Speaker, advisory board member and/or clinical investigator. Pfizer – Speaker, advisory board member and/or clinical investigator. Sandoz – Speaker, advisory board member and/or clinical investigator. Sanofi – Speaker, advisory board member and/or clinical investigator. Takeda – Speaker, advisory board member and/or clinical investigator. Theradiag – Speaker, advisory board member and/or clinical investigator.
Mark Samaan: Abbvie – Advisor or Review Panel Member, lecture fees. Bristol Myers Squibb – Advisor or Review Panel Member, lecture fees. Galapagos – Advisor or Review Panel Member, lecture fees. Janssen – Advisor or Review Panel Member, lecture fees. Lilly – lecture fees. MSD – Advisor or Review Panel Member, lecture fees. Pfizer – Advisor or Review Panel Member. Samsung Biopis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member. Sanofi – Advisor or Review Panel Member. STADA – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, lecture fees. Tilliots – Advisor or Review Panel Member.
Byong Duk Ye: Abbvie Korea – Consultant, Speakers Bureau. BMS Pharmaceutical Korea Ltd – Consultant, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Chong Kun Dang Pharm – Consultant. CJ Red BIO – Consultant. Cornerstones Health – Speakers Bureau. Curacle – Consultant, Speakers Bureau. Daewoong Pharm – Consultant, Speakers Bureau. Dong-A ST – Consultant. Eisai Korea – Speakers Bureau. Eli Lilly and Company Korea – Consultant. Ferring Korea – Consultant, Speakers Bureau. Imscout – Consultant. IQVIA – Consultant, Speakers Bureau. JEIL Pharmaceutical Co. – Consultant. Johnson & Johnson – Consultant. Johnson & Johnson Korea – Consultant, Speakers Bureau. Kangstem Biotech – Consultant. Korea Otsuka Pharm – Consultant. Korea United Pharm – Consultant. Medtronic Korea – Consultant. NanoEntek – Consultant. ORGANOIDSCIENCES Ltd – Consultant. Pfizer Korea – Consultant, Grant/Research Support, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Takeda – Consultant. Takeda Korea – Consultant, Speakers Bureau. Yuhan – Consultant.
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Nicole Shipitofsky: Johnson & Johnson – Employee.
Dwiti Pandya: Johnson & Johnson – Employee.
Ye Miao: Johnson & Johnson – Employee.
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Axel Dignass: Abbvie – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Abivax – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Johnson & Johnson – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Lilly – Consultant. Materia – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Prima – Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Stada – Consultant. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tilliots – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Tadakazu Hisamatsu, MD, PhD1, Julián Panés, MD2, Fernando Magro, MD, PhD3, Gary R.. Lichtenstein, MD, FACG4, Jessica R.. Allegretti, MD, MPH5, Brian Bressler, MD, MS6, Waqqas Afif, MD7, Mark A. Samaan, MD, MBBS8, Byong Duk Ye, MD, PhD9, Shadi Yarandi, MD10, Matthew Germinaro, MD10, Nicole Shipitofsky, PharmD10, Dwiti Pandya, MS10, Ye Miao, MS10, Hongyan Zhang, 10, Axel Dignass, MD, PhD11, Bruce E. Sands, MD, MS, FACG12. P3189 - Maintenance of Endoscopic and Histologic Efficacy With Guselkumab for Ulcerative Colitis at Week 92 of the QUASAR Long-Term Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Tadakazu Hisamatsu, MD, PhD1, Julián Panés, MD2, Fernando Magro, MD, PhD3, Gary R.. Lichtenstein, MD, FACG4, Jessica R.. Allegretti, MD, MPH5, Brian Bressler, MD, MS6, Waqqas Afif, MD7, Mark A. Samaan, MD, MBBS8, Byong Duk Ye, MD, PhD9, Shadi Yarandi, MD10, Matthew Germinaro, MD10, Nicole Shipitofsky, PharmD10, Dwiti Pandya, MS10, Ye Miao, MS10, Hongyan Zhang, 10, Axel Dignass, MD, PhD11, Bruce E. Sands, MD, MS, FACG12
1Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 2Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 3University of Porto, Porto, Porto, Portugal; 4University of Pennsylvania, Philadelphia, PA; 5Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 6University of British Columbia, Vancouver, BC, Canada; 7Division of Gastroenterology, McGill University Health Care, Montreal, PQ, Canada; 8Inflammatory Bowel Disease Unit, Guy’s and St Thomas’ NHS Foundation Trust, London, England, United Kingdom; 9University of Ulsan College of Medicine, Asan Medical Center, Seoul, Seoul-t'ukpyolsi, Republic of Korea; 10Johnson & Johnson, Spring House, PA; 11Agaplesion Markus Hospital, Frankfurt, Hessen, Germany; 12Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY
Introduction: Guselkumab (GUS) is a dual-acting IL-23p19 subunit inhibitor that potently neutralizes IL-23 and binds to CD64, a receptor on cells that produce IL-23. In the QUASAR studies, GUS intravenous (IV) induction and subcutaneous (SC) maintenance demonstrated symptomatic, endoscopic, and histologic efficacy through 2 years.1,2 Here, we describe maintenance of endoscopic and histologic efficacy in the long-term extension (LTE).
Methods: At maintenance week (W) 0, GUS IV induction responders were randomized 1:1:1 to GUS 100 mg SC every 8 weeks (q8w), GUS 200 mg SC q4w or placebo (GUS withdrawal). Participants (pts) completing the W44 visit could enter the LTE and continue the treatment regimen they were receiving at W44. Placebo pts discontinued after study unblinding. Efficacy data for randomized pts who continued to receive their GUS regimen assigned at W0 in the LTE were analyzed using 2 methods: 1) nonresponder imputation (NRI) accounting for pts with treatment failure or missing data, and 2) “as observed.”
Results: Overall, 303 randomized pts (155 for 100 mg q8w and 148 for 200 mg q4w) continued their GUS treatment assigned at W0 in the LTE. Of pts who achieved endoscopic improvement at W44, 87.2% of GUS 100 mg q8w pts and 80.2% GUS 200 mg q4w pts maintained endoscopic improvement through W92 (NRI analysis; Figure 1). Of pts who achieved histologic improvement at W44, 77.9% and 75.2%, respectively, maintained histologic improvement at W92. Of pts who achieved histo-endoscopic mucosal improvement (HEMI) at W44, 73.5% and 75.5%, respectively, maintained HEMI through W92. Of pts who were in histologic remission at W44, 71.2% maintained histologic remission at W92 in both GUS groups. Results of the as-observed analysis were consistent with the NRI analysis; the dropout rate was low among GUS-treated pts in the LTE. Proportions of pts who maintained endoscopic and histologic outcomes from W0 to W92 are presented in Figure 2.
Discussion: High rates of long-term sustained endoscopic and histologic endpoints were observed at W92 of the LTE with both GUS dose regimens.
References: 1. Rubin DT, et al. Lancet. 2025;405:33-49; 2. Lichtenstein GR, et al. Presented at DDW 2025; May 4–6, 2025; San Diego, CA.

Figure: Figure 1. Maintenance of endoscopic and histological efficacy with GUS from W44 to W92 in the QUASAR LTE study based on NRI and as-observed analysis.
Includes pts with modified Mayo score of 5-9 at induction baseline who achieved clinical response to GUS IV induction and were randomized to receive GUS maintenance treatment and did not experience a dose adjustment from W8 through W32.
Proportions of pts reflect pts achieving the endpoint at W92 among those who achieved the corresponding endpoint at W44.
Endoscopic improvement is defined as an endoscopic subscore of 0 or 1. Histologic improvement is defined as neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system. HEMI is defined as achieving a combination of histologic improvement and endoscopic improvement. Histologic remission is defined as the absence of neutrophils from the mucosa (both lamina propria and epithelium), no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system (equivalent to a Robarts Histopathology Index ≤2b, with subscores of 0 for lamina propria neutrophils and neutrophils in the epithelium and without ulcers or erosion).
CI=Confidence interval; GUS=Guselkumab; HEMI=histologic-endoscopic mucosal improvement; IV=Intravenous; LTE=Long-term extension; NRI=Nonresponder imputation; pts=Participants; q4w=Every 4 weeks; q8w=Every 8 weeks; SC=Subcutaneous; W=Week.

Figure: Figure 2. Maintenance of endoscopic and histological efficacy with GUS from W0 to W92 in the QUASAR LTE study based on NRI and as-observed analysis.
Includes pts with modified Mayo score of 5-9 at induction baseline who achieved clinical response to GUS IV induction and were randomized to receive GUS maintenance treatment and did not experience a dose adjustment from W8 through W32.
Proportions of pts reflect pts achieving the endpoint at W92 among those who achieved the corresponding endpoint at W0.
Endoscopic improvement is defined as an endoscopic subscore of 0 or 1. Histologic improvement is defined as neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system. HEMI is defined as achieving a combination of histologic improvement and endoscopic improvement. Histologic remission is defined as the absence of neutrophils from the mucosa (both lamina propria and epithelium), no crypt destruction, and no erosions, ulcerations or granulation tissue according to the Geboes grading system (equivalent to a Robarts Histopathology Index ≤2b, with subscores of 0 for lamina propria neutrophils and neutrophils in the epithelium and without ulcers or erosion).
CI=Confidence interval; GUS=Guselkumab; HEMI=histologic-endoscopic mucosal improvement; IV=Intravenous; LTE=Long-term extension; NRI=Nonresponder imputation; pts=Participants; q4w=Every 4 weeks; q8w=Every 8 weeks; SC=Subcutaneous; W=Week.
Disclosures:
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Julián Panés: AbbVie – Consultant. Alimentiv – Consultant, Data safety monitoring board. Athos – Consultant. Atomwise – Consultant. Boehringer Ingelheim – Consultant. Celsius – Consultant. Ferring – Consultant. Galapagos – Consultant. Genentech/Roche – Consultant. GlaxoSmithKline – Consultant. Johnson & Johnson – Consultant. Mirum – Consultant, Data safety monitoring board. Nimbus – Consultant. Pfizer – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist – Consultant. Revolo – Consultant. Sanofi – Consultant, Data safety monitoring board. Sorriso – Consultant, Data safety monitoring board. Surrozen – Consultant, Data safety monitoring board. Takeda – Consultant. Wasserman – Consultant.
Fernando Magro: AbbVie – Speakers Bureau. Arena Pharmaceuticals – Speakers Bureau. Biogen – Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring Pharmaceuticals – Speakers Bureau. Hospira – Speakers Bureau. Janssen – Speakers Bureau. Laboratórios Vitoria – Speakers Bureau. Merck Sharp & Dohme – Speakers Bureau. Pfizer – Speakers Bureau. Sandoz – Speakers Bureau. Takeda – Speakers Bureau. UCB Pharma – Speakers Bureau. Vifor Pharma – Speakers Bureau.
Gary Lichtenstein: Abbvie – Consultant. American College of Gastroenterology – honorarium as associate editor of American Journal of Gastroenterology. Celgene – Consultant, Grant/Research Support. CellCeutrix – Consultant. Clinical Advances in Gastroenterology – Honorarium - Associate Editor. Eli Lilly – Consultant, DSMB. Ferring – Consultant. Gastroenterology and Hepatology [Gastro-Hep Communications] – Honorarium - Editor. Gilead – Consultant. Johnson & Johnson – Consultant. Luitpold/American Regent – Consultant, Honorarium - CME programs. McMahon Publishing – Honorarium - Author. Merck – Consultant, Honorarium - CME programs. Orthobiotech – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Romark – Consultant, Honorarium - CME programs. Salix/Valeant – Consultant, Grant/Research Support. Shire – Consultant, Grant/Research Support. SLACK – Book royalty. Springer – Honorarium - Editor. Takeda – Consultant, Funding to institution [IBD fellow education]. UCB – Consultant, Grant/Research Support. Up-To-Date – Honorarium - Author. Vindico – CME. Virgo – Consultant, Stock Options.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Brian Bressler: Abbvie – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Amgen – Advisor or Review Panel Member, Grant/Research Support. AMT – Advisor or Review Panel Member. Bausch Health – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Celgene – Advisor or Review Panel Member. Celltrion – Advisor or Review Panel Member. Eupraxia Fresenius Kabi – Advisor or Review Panel Member. Ferring – Advisor or Review Panel Member, Speakers Bureau. Genentech/Roche – Advisor or Review Panel Member, Grant/Research Support. Gilead – Advisor or Review Panel Member. GlaxoSmithKline – Grant/Research Support. Iterative Scopes – Advisor or Review Panel Member. Jamp – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Mylan – Advisor or Review Panel Member. Novartis – Advisor or Review Panel Member, Speakers Bureau. Organaon – Advisor or Review Panel Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Takeda – Advisor or Review Panel Member, Speakers Bureau. Viatris – Advisor or Review Panel Member.
Waqqas Afif: Abbvie – Speaker, advisory board member and/or clinical investigator. Amgen – Speaker, advisory board member and/or clinical investigator. Eli Lilly – Speaker, advisory board member and/or clinical investigator. Johnson & Johnson – Speaker, advisory board member and/or clinical investigator. Merck – Speaker, advisory board member and/or clinical investigator. Novartis – Speaker, advisory board member and/or clinical investigator. Pfizer – Speaker, advisory board member and/or clinical investigator. Sandoz – Speaker, advisory board member and/or clinical investigator. Sanofi – Speaker, advisory board member and/or clinical investigator. Takeda – Speaker, advisory board member and/or clinical investigator. Theradiag – Speaker, advisory board member and/or clinical investigator.
Mark Samaan: Abbvie – Advisor or Review Panel Member, lecture fees. Bristol Myers Squibb – Advisor or Review Panel Member, lecture fees. Galapagos – Advisor or Review Panel Member, lecture fees. Janssen – Advisor or Review Panel Member, lecture fees. Lilly – lecture fees. MSD – Advisor or Review Panel Member, lecture fees. Pfizer – Advisor or Review Panel Member. Samsung Biopis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member. Sanofi – Advisor or Review Panel Member. STADA – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, lecture fees. Tilliots – Advisor or Review Panel Member.
Byong Duk Ye: Abbvie Korea – Consultant, Speakers Bureau. BMS Pharmaceutical Korea Ltd – Consultant, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Chong Kun Dang Pharm – Consultant. CJ Red BIO – Consultant. Cornerstones Health – Speakers Bureau. Curacle – Consultant, Speakers Bureau. Daewoong Pharm – Consultant, Speakers Bureau. Dong-A ST – Consultant. Eisai Korea – Speakers Bureau. Eli Lilly and Company Korea – Consultant. Ferring Korea – Consultant, Speakers Bureau. Imscout – Consultant. IQVIA – Consultant, Speakers Bureau. JEIL Pharmaceutical Co. – Consultant. Johnson & Johnson – Consultant. Johnson & Johnson Korea – Consultant, Speakers Bureau. Kangstem Biotech – Consultant. Korea Otsuka Pharm – Consultant. Korea United Pharm – Consultant. Medtronic Korea – Consultant. NanoEntek – Consultant. ORGANOIDSCIENCES Ltd – Consultant. Pfizer Korea – Consultant, Grant/Research Support, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Takeda – Consultant. Takeda Korea – Consultant, Speakers Bureau. Yuhan – Consultant.
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Nicole Shipitofsky: Johnson & Johnson – Employee.
Dwiti Pandya: Johnson & Johnson – Employee.
Ye Miao: Johnson & Johnson – Employee.
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Axel Dignass: Abbvie – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Abivax – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Johnson & Johnson – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Lilly – Consultant. Materia – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Prima – Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Stada – Consultant. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tilliots – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Tadakazu Hisamatsu, MD, PhD1, Julián Panés, MD2, Fernando Magro, MD, PhD3, Gary R.. Lichtenstein, MD, FACG4, Jessica R.. Allegretti, MD, MPH5, Brian Bressler, MD, MS6, Waqqas Afif, MD7, Mark A. Samaan, MD, MBBS8, Byong Duk Ye, MD, PhD9, Shadi Yarandi, MD10, Matthew Germinaro, MD10, Nicole Shipitofsky, PharmD10, Dwiti Pandya, MS10, Ye Miao, MS10, Hongyan Zhang, 10, Axel Dignass, MD, PhD11, Bruce E. Sands, MD, MS, FACG12. P3189 - Maintenance of Endoscopic and Histologic Efficacy With Guselkumab for Ulcerative Colitis at Week 92 of the QUASAR Long-Term Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

