Monday Poster Session
Category: IBD
P3177 - Mirikizumab Improves Quality of Life in Patients With Crohn’s Disease Up to 2 Years of Treatment: Results From the VIVID-2 Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- AV
Aisha Vadhariya
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
Sarah Glover, DO1, Millie D. Long, MD, FACG2, Aisha Vadhariya, 3, Yiying Brogan, 3, Guanglei Yu, PhD4, Jianmin Wu, 3, Ailsa Hart, BA, BMBCh, PhD5, Lisa Malter, MD, FACG6
1Tulane University, New Orleans, LA; 2Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 3Eli Lilly and Company, Indianapolis, IN; 4Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 5London North-West University Healthcare NHS Trust, London, England, United Kingdom; 6Division of Gastroenterology, Department of Medicine, NYU Langone Health, New York, NY
Introduction: Mirikizumab is a humanized IgG4 monoclonal, anti-interleukin-23p19 antibody. VIVID-2 (NCT04232553), an ongoing open-label study, has demonstrated sustained clinical and endoscopic efficacy at 2 years of treatment of mirikizumab for Crohn’s disease (CD). Here we present the long-term quality of life (QoL) up to 2 years from VIVID-2.
Methods: Patients enrolled in the phase 3 VIVID-1 study who achieved an endoscopic response to mirikizumab continued maintenance treatment in VIVID-2, while endoscopic non-responders received mirikizumab induction therapy followed by maintenance treatment. Quality of life was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ). The proportion of patients who achieved IBDQ response/remission, as well as mean changes from VIVID-1 baseline in IBDQ total scores at W64 and104 were reported. EQ-5D-5L Visual Analog Scale (VAS) scores (range 0-100, with 100 being best imaginable health state) were assessed at W64. Work productivity was assessed at W64 using Work Productivity and Activity Impairment Questionnaire in Crohn's Disease (WPAI:CD) (4 scores including absenteeism, presenteeism, work productivity loss and activity impairment are derived as percentages with higher values indicating greater impairment). Modified non-responder imputation (mNRI) was used for binary missing data imputation, and modified baseline observation carried forward (mBOCF) was used for continuous missing data imputation. Estimates of mean changes from baseline were calculated with analysis of covariance (ANCOVA).
Results: Analysis of endoscopic responders showed IBDQ response and IBDQ remission were maintained through W104 (85.0% and 71.3%, respectively) while endoscopic non-responders also maintained IBDQ response and remission through the same time (72.6% and 60.8%, respectively). (Table 1) Mean change from baseline in IBDQ total score was maintained through W104 for endoscopic responders and non-responders (least squares mean (standard error); 57.2 (2.1) and 46.8 (2.4), respectively). Similarly, EQ-5D-5L VAS improvement was observed for both groups at W64 (27.0 (1.0) and 22.3 (1.2), respectively). WPAI:CD improvements in both groups ranged from −13.9 (1.8) to −34.3 (1.5) for the different scores collected at W64.
Discussion: Patients continuing mirikizumab maintenance and those receiving reinduction achieved meaningful long-term QoL improvement that was maintained at 2 years of treatment.
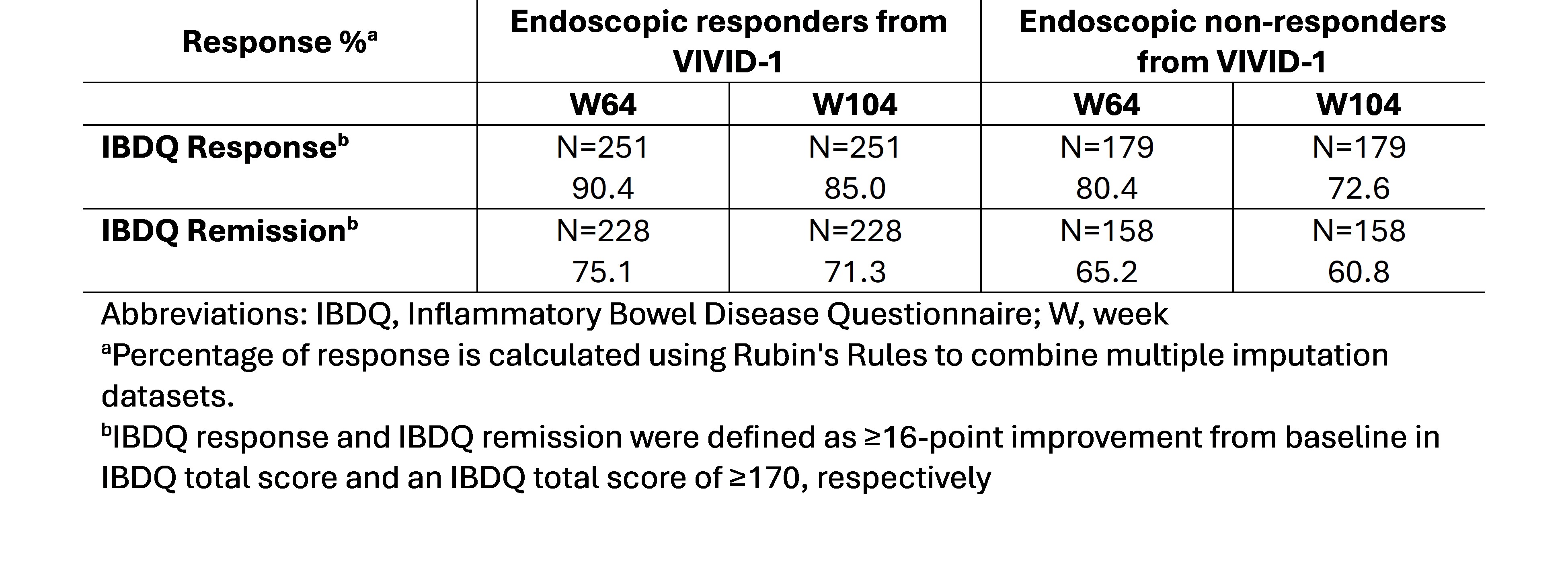
Figure: IBDQ Response and Remission Among Patients Receiving Mirikizumab Through W104
Disclosures:
Sarah Glover: Janssen – Consultant.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Yiying Brogan: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Ailsa Hart: AbbVie – Advisory Committee/Board Member, Lecturer. Bristol Myers Squibb – Advisory Committee/Board Member, Lecturer. Celltrion – Advisory Committee/Board Member, Lecturer. Falk – Advisory Committee/Board Member, Lecturer. Galapagos – Advisory Committee/Board Member, Lecturer. GSK – Advisory Committee/Board Member, Lecturer. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Lecturer. MSD – Advisory Committee/Board Member, Lecturer. Pfizer – Advisory Committee/Board Member, Lecturer. Takeda – Advisory Committee/Board Member, Lecturer.
Lisa Malter: AbbVie – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Janssen – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Pharmacosmos – Consultant.
Sarah Glover, DO1, Millie D. Long, MD, FACG2, Aisha Vadhariya, 3, Yiying Brogan, 3, Guanglei Yu, PhD4, Jianmin Wu, 3, Ailsa Hart, BA, BMBCh, PhD5, Lisa Malter, MD, FACG6. P3177 - Mirikizumab Improves Quality of Life in Patients With Crohn’s Disease Up to 2 Years of Treatment: Results From the VIVID-2 Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Tulane University, New Orleans, LA; 2Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 3Eli Lilly and Company, Indianapolis, IN; 4Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 5London North-West University Healthcare NHS Trust, London, England, United Kingdom; 6Division of Gastroenterology, Department of Medicine, NYU Langone Health, New York, NY
Introduction: Mirikizumab is a humanized IgG4 monoclonal, anti-interleukin-23p19 antibody. VIVID-2 (NCT04232553), an ongoing open-label study, has demonstrated sustained clinical and endoscopic efficacy at 2 years of treatment of mirikizumab for Crohn’s disease (CD). Here we present the long-term quality of life (QoL) up to 2 years from VIVID-2.
Methods: Patients enrolled in the phase 3 VIVID-1 study who achieved an endoscopic response to mirikizumab continued maintenance treatment in VIVID-2, while endoscopic non-responders received mirikizumab induction therapy followed by maintenance treatment. Quality of life was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ). The proportion of patients who achieved IBDQ response/remission, as well as mean changes from VIVID-1 baseline in IBDQ total scores at W64 and104 were reported. EQ-5D-5L Visual Analog Scale (VAS) scores (range 0-100, with 100 being best imaginable health state) were assessed at W64. Work productivity was assessed at W64 using Work Productivity and Activity Impairment Questionnaire in Crohn's Disease (WPAI:CD) (4 scores including absenteeism, presenteeism, work productivity loss and activity impairment are derived as percentages with higher values indicating greater impairment). Modified non-responder imputation (mNRI) was used for binary missing data imputation, and modified baseline observation carried forward (mBOCF) was used for continuous missing data imputation. Estimates of mean changes from baseline were calculated with analysis of covariance (ANCOVA).
Results: Analysis of endoscopic responders showed IBDQ response and IBDQ remission were maintained through W104 (85.0% and 71.3%, respectively) while endoscopic non-responders also maintained IBDQ response and remission through the same time (72.6% and 60.8%, respectively). (Table 1) Mean change from baseline in IBDQ total score was maintained through W104 for endoscopic responders and non-responders (least squares mean (standard error); 57.2 (2.1) and 46.8 (2.4), respectively). Similarly, EQ-5D-5L VAS improvement was observed for both groups at W64 (27.0 (1.0) and 22.3 (1.2), respectively). WPAI:CD improvements in both groups ranged from −13.9 (1.8) to −34.3 (1.5) for the different scores collected at W64.
Discussion: Patients continuing mirikizumab maintenance and those receiving reinduction achieved meaningful long-term QoL improvement that was maintained at 2 years of treatment.

Figure: IBDQ Response and Remission Among Patients Receiving Mirikizumab Through W104
Disclosures:
Sarah Glover: Janssen – Consultant.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Yiying Brogan: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Ailsa Hart: AbbVie – Advisory Committee/Board Member, Lecturer. Bristol Myers Squibb – Advisory Committee/Board Member, Lecturer. Celltrion – Advisory Committee/Board Member, Lecturer. Falk – Advisory Committee/Board Member, Lecturer. Galapagos – Advisory Committee/Board Member, Lecturer. GSK – Advisory Committee/Board Member, Lecturer. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Lecturer. MSD – Advisory Committee/Board Member, Lecturer. Pfizer – Advisory Committee/Board Member, Lecturer. Takeda – Advisory Committee/Board Member, Lecturer.
Lisa Malter: AbbVie – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Janssen – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Pharmacosmos – Consultant.
Sarah Glover, DO1, Millie D. Long, MD, FACG2, Aisha Vadhariya, 3, Yiying Brogan, 3, Guanglei Yu, PhD4, Jianmin Wu, 3, Ailsa Hart, BA, BMBCh, PhD5, Lisa Malter, MD, FACG6. P3177 - Mirikizumab Improves Quality of Life in Patients With Crohn’s Disease Up to 2 Years of Treatment: Results From the VIVID-2 Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
