Monday Poster Session
Category: IBD
P3167 - Inhibition of Structural Damage Progression With Guselkumab in Participants With Active Psoriatic Arthritis: Results Through Week 24 of the Phase 3b, Randomized, Double-Blind, Placebo-Controlled APEX Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Soumya Chakravarty, MD, PhD
Johnson & Johnson; Drexel University College of Medicine
Horsham, PA
Presenting Author(s)
Philip J. Mease, MD1, Christopher T. Ritchlin, MD, MPH2, Laura C. Coates, MBChB, PhD3, Alexa P. Kollmeier, MD4, Bei Zhou, PhD5, Yusang Jiang, MA5, Karen Bensley, BSN5, Koeun Im, BSc6, Rattandeep Batra, MPharm7, Soumya Chakravarty, MD, PhD8, Proton Rahman, MD9, Désirée van der Heijde, MD, PhD10
1Rheumatology Research, Providence Swedish Medical Center; University of Washington School of Medicine, Seattle, WA; 2Department of Medicine, Allergy/Immunology and Rheumatology, University of Rochester Medical Center, Rochester, NY; 3Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Botnar Research Centre,, Oxford, England, United Kingdom; 4Johnson & Johnson, San Diego, CA; 5Johnson & Johnson, Spring House, PA; 6Johnson & Johnson, Cambridge, MA; 7Johnson & Johnson, Toronto, ON, Canada; 8Johnson & Johnson; Drexel University College of Medicine, Horsham, PA; 9Craig L Dobbin Genetics Research Centre, Faculty of Medicine, Division of Rheumatology, Memorial University of Newfoundland, St. John's, NF, Canada; 10Leiden University Medical Center, Leiden, Zuid-Holland, Netherlands
Introduction: Extraintestinal manifestations(EIMs), eg, joint involvement and psoriatic arthritis(PsA), are common in pts with IBD and may be associated with more severe IBD. Guselkumab(GUS), a dual-acting IL-23p19 subunit inhibitor, is approved to treat CD, UC, PsA, and psoriasis. No studies have prospectively evaluated the efficacy of GUS on EIMs in IBD; data from psoriatic studies may be informative. The Phase 3b, randomized, double-blind, placebo-controlled APEX study evaluated clinical and radiographic outcomes in GUS-treated pts with active PsA. Here, we report Week 24(W24) results.
Methods: APEX enrolled biologic naïve adults with active PsA (≥3 tender & ≥3 swollen joints; C-reactive protein ≥0.3mg/dL) and ≥2 erosive joints on radiographs of hands and feet despite previous non-biologic DMARDs, apremilast, or NSAIDs. Pts were randomized 5:7:7 to SC GUS100mg q4w; GUS100mg at W0/4, then q8w; or PBO q4w. Multiplicity-controlled endpoints were the proportion of pts achieving ≥20% improvement in American College of Rheumatology response criteria(ACR20)(primary) and the mean change from baseline(BL) in PsA-modified van der Heijde-Sharp(vdH-S) score at W24(secondary). Efficacy analyses included all randomized pts except those from sites unable to support key study operations (N=1020; q4w: 273, q8w: 371, PBO: 376); safety analyses included all treated pts through W24(N=1054).
Results: BL characteristics were similar across groups. Mean duration of PsA(7.3yrs), PsA-modified vdH-S total(27.0) and erosion(13.5) scores, and tender(20.7) and swollen(11.9) joint counts indicated established, highly active joint disease. Primary and major secondary endpoints were met. Significantly greater proportions of GUSq4w(67%), and q8w(68%) pts vs PBO(47%) achieved ACR20 at W24(both P< 0.001; Fig 1A). Pts receiving GUSq4w and q8w had significantly less radiographic progression vs PBO at W24(LSM changes in PsA-modified vdH-S scores: 0.55, 0.54 vs 1.35; P=0.002,< 0.001, respectively; Fig 1B). Similar response patterns occurred at W24 for changes in joint space narrowing and erosion scores, pts with no radiographic progression, and other clinical outcomes(Table). AE rates were similar among groups (37-42%); SAE rates were low (2-3%), and no new safety signals were identified.
Discussion: GUS improved arthritis and inhibited structural damage progression in pts with active PsA. Findings may inform treatment decisions in pts with IBD with articular EIMs related to psoriatic disease.
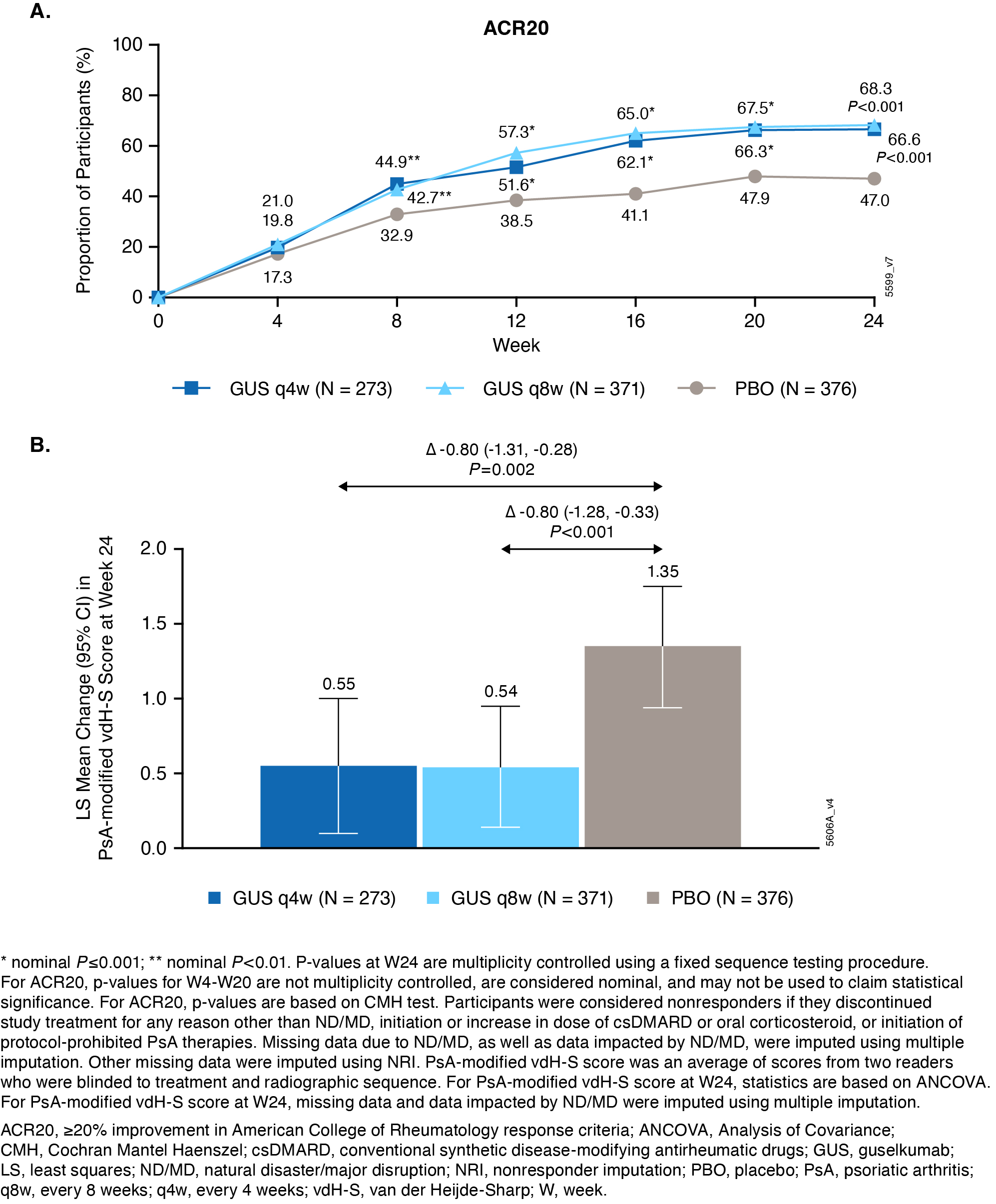
Figure: Figure 1. Proportions of participants achieving ACR20 over time through Week 24 (A) and LS mean change from baseline to Week 24 in PsA-modified vdH-S score (Reading Session 1; B)
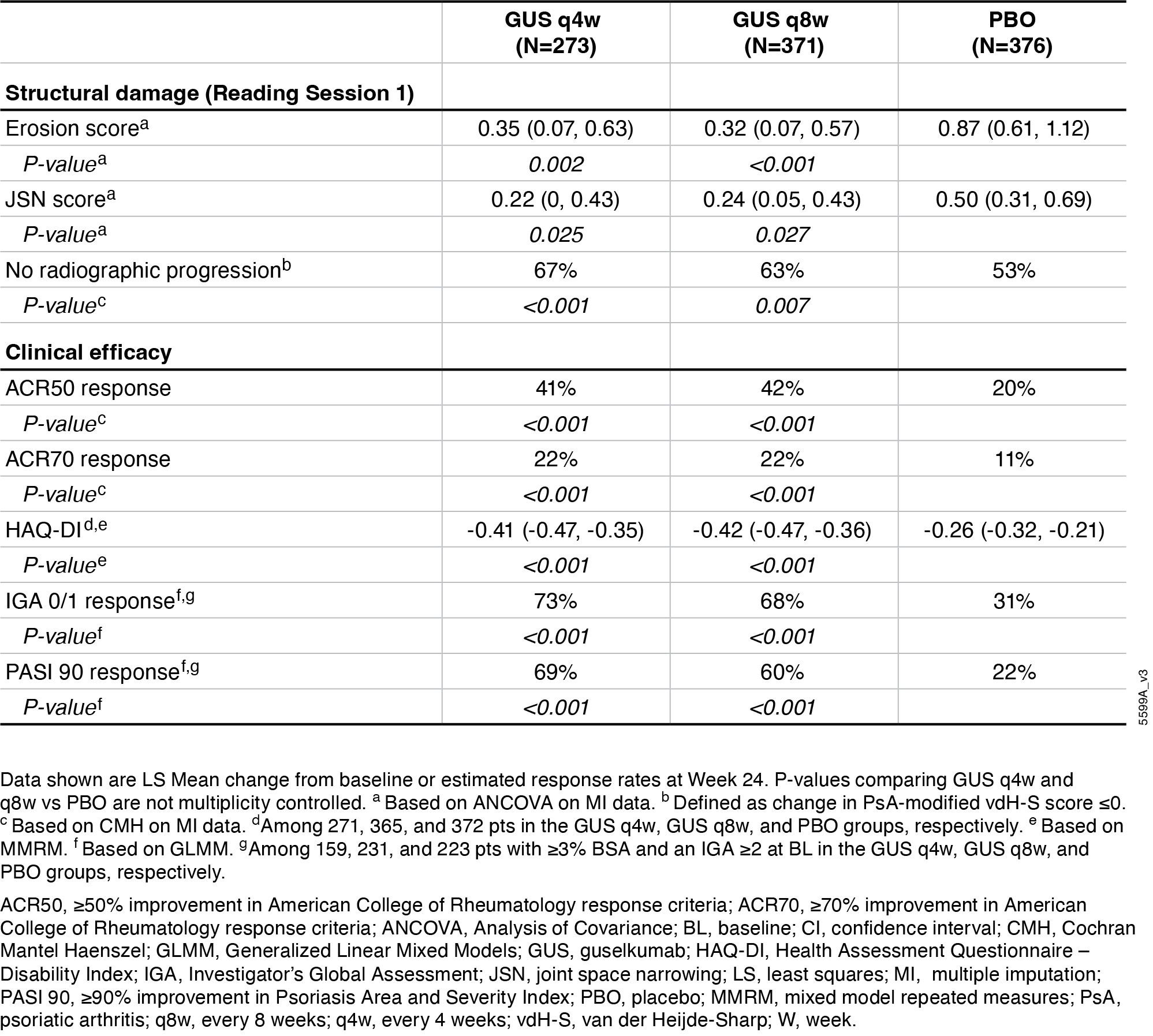
Figure: Table. Summary of other structural damage and clinical efficacy endpoints at Week 24
Disclosures:
Philip Mease: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Acelyrin – Consultant, Grant/Research Support. Amgen – Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Consultant, Grant/Research Support. Eli Lilly – Consultant, Grant/Research Support, Speakers Bureau. Immagene – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Novartis – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. Ventyx – Consultant.
Christopher Ritchlin: AbbVie – Consultant, Grant/Research Support. Amgen – Consultant, Grant/Research Support. Eli Lilly – Consultant. Gilead – Consultant. Johnson & Johnson – Consultant. Novartis – Consultant. Pfizer – Consultant. UCB – Consultant, Grant/Research Support.
Laura Coates: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Amgen – Consultant, Grant/Research Support, Speakers Bureau. Biogen – Speakers Bureau. Bristol Myers Squibb – Consultant. Celgene – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant, Grant/Research Support, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GlaxoSmithKline – Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Medac – Speakers Bureau. Moonlake – Consultant. Novartis – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau.
Alexa Kollmeier: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Bei Zhou: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Yusang Jiang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Karen Bensley: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Koeun Im: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rattandeep Batra: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Soumya Chakravarty: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Proton Rahman: AbbVie – Consultant. Amgen – Consultant. Briston Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Meeting attendance/travel support. Merck – Consultant. Novartis – Consultant, Grant/Research Support. Pfizer – Consultant. UCB – Consultant.
Désirée van der Heijde: AbbVie – Consultant. Alfasigma – Consultant. Annals of Rheumatic Diseases – Associate Editor. ArgenX – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly – Consultant. Grey-Wolf Therapeutics – Consultant. Imaging Rheumatology BV – Director. Johnson & Johnson – Consultant. Journal of Rheumatology – Editorial board member. Novartis – Consultant. Pfizer – Consultant. Takda – Consultant. UCB – Consultant.
Philip J. Mease, MD1, Christopher T. Ritchlin, MD, MPH2, Laura C. Coates, MBChB, PhD3, Alexa P. Kollmeier, MD4, Bei Zhou, PhD5, Yusang Jiang, MA5, Karen Bensley, BSN5, Koeun Im, BSc6, Rattandeep Batra, MPharm7, Soumya Chakravarty, MD, PhD8, Proton Rahman, MD9, Désirée van der Heijde, MD, PhD10. P3167 - Inhibition of Structural Damage Progression With Guselkumab in Participants With Active Psoriatic Arthritis: Results Through Week 24 of the Phase 3b, Randomized, Double-Blind, Placebo-Controlled APEX Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rheumatology Research, Providence Swedish Medical Center; University of Washington School of Medicine, Seattle, WA; 2Department of Medicine, Allergy/Immunology and Rheumatology, University of Rochester Medical Center, Rochester, NY; 3Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Botnar Research Centre,, Oxford, England, United Kingdom; 4Johnson & Johnson, San Diego, CA; 5Johnson & Johnson, Spring House, PA; 6Johnson & Johnson, Cambridge, MA; 7Johnson & Johnson, Toronto, ON, Canada; 8Johnson & Johnson; Drexel University College of Medicine, Horsham, PA; 9Craig L Dobbin Genetics Research Centre, Faculty of Medicine, Division of Rheumatology, Memorial University of Newfoundland, St. John's, NF, Canada; 10Leiden University Medical Center, Leiden, Zuid-Holland, Netherlands
Introduction: Extraintestinal manifestations(EIMs), eg, joint involvement and psoriatic arthritis(PsA), are common in pts with IBD and may be associated with more severe IBD. Guselkumab(GUS), a dual-acting IL-23p19 subunit inhibitor, is approved to treat CD, UC, PsA, and psoriasis. No studies have prospectively evaluated the efficacy of GUS on EIMs in IBD; data from psoriatic studies may be informative. The Phase 3b, randomized, double-blind, placebo-controlled APEX study evaluated clinical and radiographic outcomes in GUS-treated pts with active PsA. Here, we report Week 24(W24) results.
Methods: APEX enrolled biologic naïve adults with active PsA (≥3 tender & ≥3 swollen joints; C-reactive protein ≥0.3mg/dL) and ≥2 erosive joints on radiographs of hands and feet despite previous non-biologic DMARDs, apremilast, or NSAIDs. Pts were randomized 5:7:7 to SC GUS100mg q4w; GUS100mg at W0/4, then q8w; or PBO q4w. Multiplicity-controlled endpoints were the proportion of pts achieving ≥20% improvement in American College of Rheumatology response criteria(ACR20)(primary) and the mean change from baseline(BL) in PsA-modified van der Heijde-Sharp(vdH-S) score at W24(secondary). Efficacy analyses included all randomized pts except those from sites unable to support key study operations (N=1020; q4w: 273, q8w: 371, PBO: 376); safety analyses included all treated pts through W24(N=1054).
Results: BL characteristics were similar across groups. Mean duration of PsA(7.3yrs), PsA-modified vdH-S total(27.0) and erosion(13.5) scores, and tender(20.7) and swollen(11.9) joint counts indicated established, highly active joint disease. Primary and major secondary endpoints were met. Significantly greater proportions of GUSq4w(67%), and q8w(68%) pts vs PBO(47%) achieved ACR20 at W24(both P< 0.001; Fig 1A). Pts receiving GUSq4w and q8w had significantly less radiographic progression vs PBO at W24(LSM changes in PsA-modified vdH-S scores: 0.55, 0.54 vs 1.35; P=0.002,< 0.001, respectively; Fig 1B). Similar response patterns occurred at W24 for changes in joint space narrowing and erosion scores, pts with no radiographic progression, and other clinical outcomes(Table). AE rates were similar among groups (37-42%); SAE rates were low (2-3%), and no new safety signals were identified.
Discussion: GUS improved arthritis and inhibited structural damage progression in pts with active PsA. Findings may inform treatment decisions in pts with IBD with articular EIMs related to psoriatic disease.

Figure: Figure 1. Proportions of participants achieving ACR20 over time through Week 24 (A) and LS mean change from baseline to Week 24 in PsA-modified vdH-S score (Reading Session 1; B)

Figure: Table. Summary of other structural damage and clinical efficacy endpoints at Week 24
Disclosures:
Philip Mease: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Acelyrin – Consultant, Grant/Research Support. Amgen – Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Consultant, Grant/Research Support. Eli Lilly – Consultant, Grant/Research Support, Speakers Bureau. Immagene – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Novartis – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. Ventyx – Consultant.
Christopher Ritchlin: AbbVie – Consultant, Grant/Research Support. Amgen – Consultant, Grant/Research Support. Eli Lilly – Consultant. Gilead – Consultant. Johnson & Johnson – Consultant. Novartis – Consultant. Pfizer – Consultant. UCB – Consultant, Grant/Research Support.
Laura Coates: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Amgen – Consultant, Grant/Research Support, Speakers Bureau. Biogen – Speakers Bureau. Bristol Myers Squibb – Consultant. Celgene – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant, Grant/Research Support, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GlaxoSmithKline – Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Medac – Speakers Bureau. Moonlake – Consultant. Novartis – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau.
Alexa Kollmeier: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Bei Zhou: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Yusang Jiang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Karen Bensley: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Koeun Im: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Rattandeep Batra: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Soumya Chakravarty: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Proton Rahman: AbbVie – Consultant. Amgen – Consultant. Briston Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Meeting attendance/travel support. Merck – Consultant. Novartis – Consultant, Grant/Research Support. Pfizer – Consultant. UCB – Consultant.
Désirée van der Heijde: AbbVie – Consultant. Alfasigma – Consultant. Annals of Rheumatic Diseases – Associate Editor. ArgenX – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly – Consultant. Grey-Wolf Therapeutics – Consultant. Imaging Rheumatology BV – Director. Johnson & Johnson – Consultant. Journal of Rheumatology – Editorial board member. Novartis – Consultant. Pfizer – Consultant. Takda – Consultant. UCB – Consultant.
Philip J. Mease, MD1, Christopher T. Ritchlin, MD, MPH2, Laura C. Coates, MBChB, PhD3, Alexa P. Kollmeier, MD4, Bei Zhou, PhD5, Yusang Jiang, MA5, Karen Bensley, BSN5, Koeun Im, BSc6, Rattandeep Batra, MPharm7, Soumya Chakravarty, MD, PhD8, Proton Rahman, MD9, Désirée van der Heijde, MD, PhD10. P3167 - Inhibition of Structural Damage Progression With Guselkumab in Participants With Active Psoriatic Arthritis: Results Through Week 24 of the Phase 3b, Randomized, Double-Blind, Placebo-Controlled APEX Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
