Monday Poster Session
Category: IBD
P3161 - Association Between Vedolizumab Trough Concentration and Clinical Outcomes in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- NN
Noma Nazir, MBBS (she/her/hers)
Detroit Medical Center/Wayne State University
Detroit, MI
Presenting Author(s)
K. Sai Varun Reddy, 1, Shree Rath, MBBS2, Ammarah Tariq, MD3, Muhammad Uzair, MBBS4, Yousef M. Husseiny, MBBCh5, Anika Goel, 6, Arun Kumar. Maloth, MBBS7, Ayesha Arshad, MBBS8, Noma Nazir, MBBS9, Mohamed N. Abohalawa, 10, Adnan Bhat, MD11, Fatima Aslam, MBBS, MD12, Ammara Tahir, MBBS13
1Kakatiya Medical College , Warangal , India, Hyderabad, Telangana, India; 2All India Institute of Medical Sciences Bhubaneswar, Bhubaneswar, Orissa, India; 3Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 4Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 5NewGiza University, New Cairo, Al Qahirah, Egypt; 6Kakatiya Medical College, Warangal, Warangal, Telangana, India; 7Kakatiya Medical College, Warangal, India, Warangal, Telangana, India; 8Dow Medical College, Karachi, Sindh, Pakistan; 9Detroit Medical Center/Wayne State University, Detroit, MI; 10Faculty of Medicine - Tanta University, El-Mahalla El-Kubra, Al Gharbiyah, Egypt; 11University of Florida, Gainesville, FL; 12Tucson Medical Center, Tucson, AZ; 13Liaquat University of Medical and Health Science, Jamshoro, Sindh, Pakistan
Introduction: Vedolizumab is a gut-selective, anti-integrin humanised monoclonal antibody that selectively targets α4β7 integrin, widely used for the treatment of inflammatory bowel diseases (IBD). The treatment response is variable, and the role of therapeutic drug monitoring (TDM) in optimising vedolizumab therapy remains unclear. This study aims to evaluate the relationship between vedolizumab trough concentrations (VTC) and clinical, endoscopic, and histologic outcomes in patients with IBD to guide the use of TDM in clinical practice.
Methods: A systematic search of PubMed/MEDLINE, Embase and Scopus was performed applying Boolean operators and MeSH keywords. PRISMA standards were followed, and the study was pre-registered on PROSPERO. Two authors independently screened 1,066 records after removing duplicates. 12 studies were included based on the inclusion criteria. Primary outcomes were clinical, endoscopic, and biologic remission; secondary outcomes were mucosal healing, steroid-free remission, response to dose optimisation, and adverse events. Risk of bias was evaluated using the NIH tool. Synthesising the data used Review Manager (RevMan v5.4.1), and pooled proportions with 95% confidence intervals were determined using a random-effects model.
Results: A total of 12 observational studies involving 3,259 patients with IBD (Crohn's disease and ulcerative colitis) were included. In the pooled proportion, clinical remission was 44% (95% CI: 13–80%), endoscopic remission 33% (95% CI: 23–43%), and steroid-free remission 39% (95% CI: 24–56%). Mucosal healing was seen in 37% of the patients (95% CI: 28–47%). The mean VTC was 18.76 μg/mL (95% CI: 12.18–25.34). The pooled response rate was 48% (95% CI: 30–67%) in patients who received dose optimisation. Serious adverse events were rare, with an overall occurrence of 8% (95% CI: 2–22%). Heterogeneity was extreme (I² = 95%), largely due to an outlier study, which was supported by sensitivity analyses and Baujat.
Discussion: This study implies a strong relationship between greater VTC and better clinical, endoscopic, and histologic results in IBD. Pooled data shows that VTC ≥18–24 μg/mL during induction and ≥12 μg/mL during maintenance are associated with remission. Optimal dosing resulted in ~50% response. Vedolizumab was very safe, with low immunogenicity and very few serious adverse events. The findings from the study support VTC-guided dosing in practice.
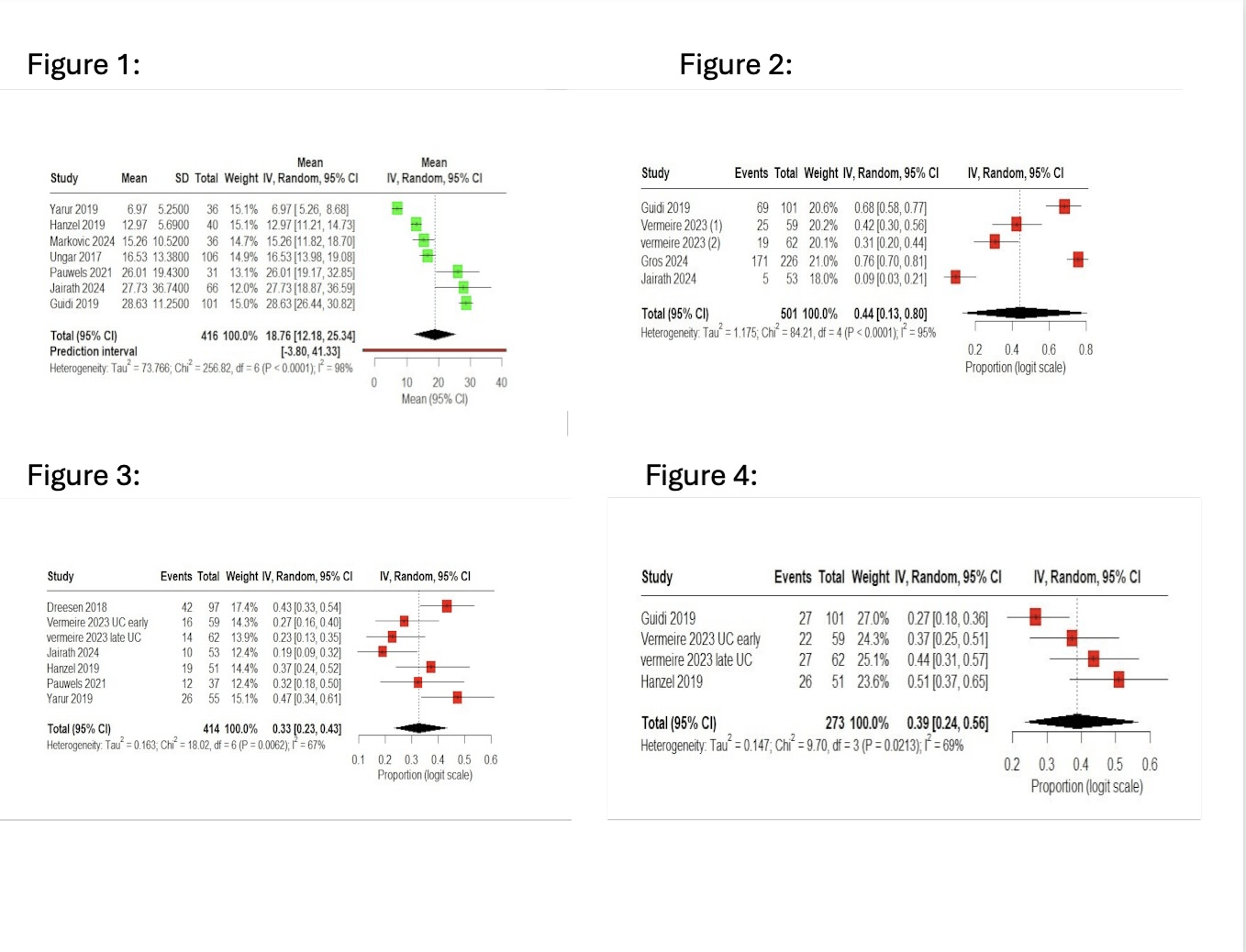
Figure: Forest Plots:
Figure 1. Forest Plot of Vedolizumab Trough Levels.
Figure 2. Forest Plot of Clinical Remission.
Figure 3. Forest Plot of Endoscopic Remission.
Figure 4. Forest Plot of Steroid Free Remission.
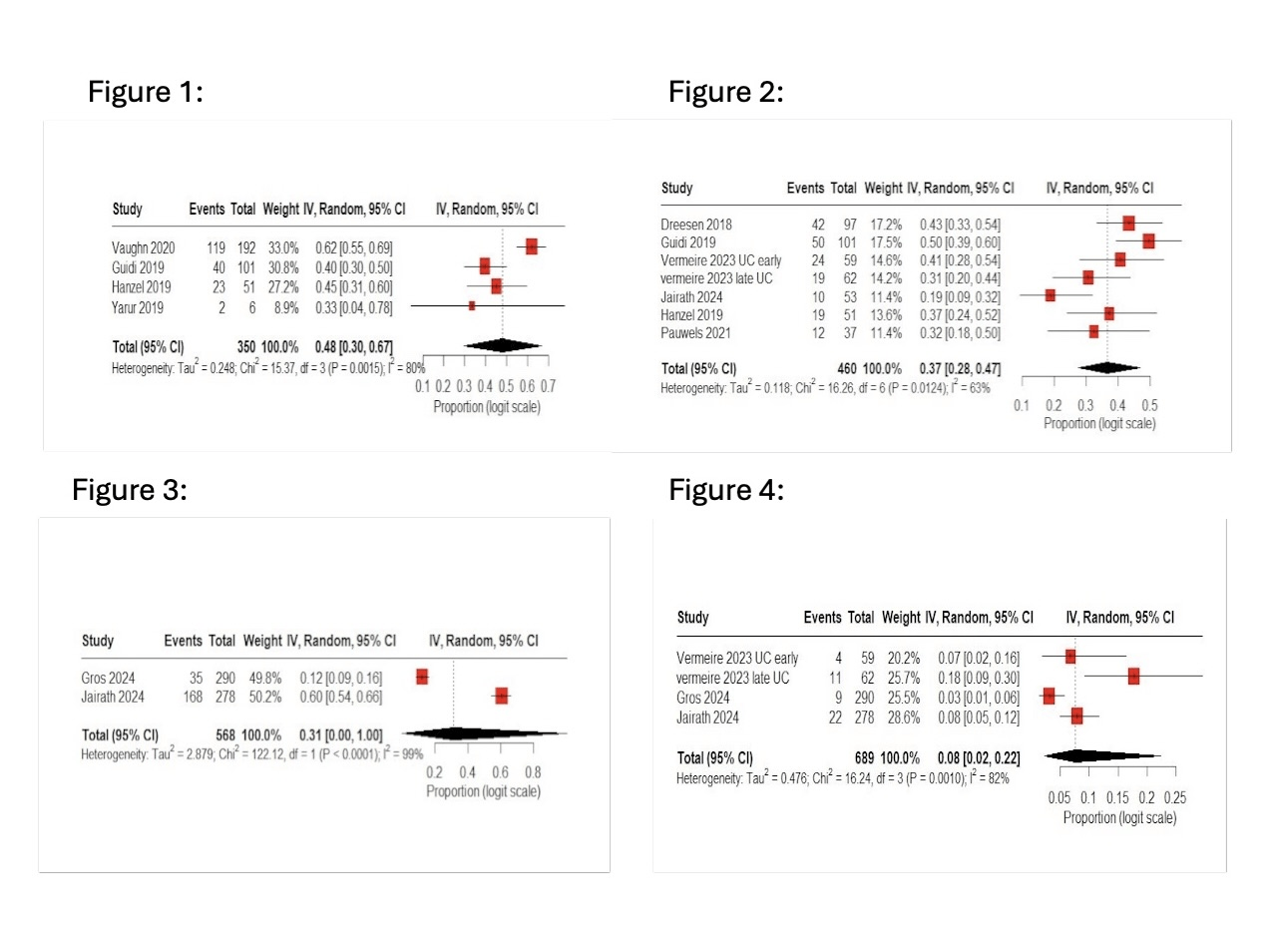
Figure: Forest Plots:
Figure 1. Forest Plot of Response to Dose Optimisation.
Figure 2. Forest Plot of Mucosal Healing.
Figure 3. Forest Plot of Any Adverse Event.
Figure 4. Forest Plot of Serious Adverse Events.
Disclosures:
K. Sai Varun Reddy indicated no relevant financial relationships.
Shree Rath indicated no relevant financial relationships.
Ammarah Tariq indicated no relevant financial relationships.
Muhammad Uzair indicated no relevant financial relationships.
Yousef M. Husseiny indicated no relevant financial relationships.
Anika Goel indicated no relevant financial relationships.
Arun Maloth indicated no relevant financial relationships.
Ayesha Arshad indicated no relevant financial relationships.
Noma Nazir indicated no relevant financial relationships.
Mohamed Abohalawa indicated no relevant financial relationships.
Adnan Bhat indicated no relevant financial relationships.
Fatima Aslam indicated no relevant financial relationships.
Ammara Tahir indicated no relevant financial relationships.
K. Sai Varun Reddy, 1, Shree Rath, MBBS2, Ammarah Tariq, MD3, Muhammad Uzair, MBBS4, Yousef M. Husseiny, MBBCh5, Anika Goel, 6, Arun Kumar. Maloth, MBBS7, Ayesha Arshad, MBBS8, Noma Nazir, MBBS9, Mohamed N. Abohalawa, 10, Adnan Bhat, MD11, Fatima Aslam, MBBS, MD12, Ammara Tahir, MBBS13. P3161 - Association Between Vedolizumab Trough Concentration and Clinical Outcomes in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Kakatiya Medical College , Warangal , India, Hyderabad, Telangana, India; 2All India Institute of Medical Sciences Bhubaneswar, Bhubaneswar, Orissa, India; 3Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 4Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 5NewGiza University, New Cairo, Al Qahirah, Egypt; 6Kakatiya Medical College, Warangal, Warangal, Telangana, India; 7Kakatiya Medical College, Warangal, India, Warangal, Telangana, India; 8Dow Medical College, Karachi, Sindh, Pakistan; 9Detroit Medical Center/Wayne State University, Detroit, MI; 10Faculty of Medicine - Tanta University, El-Mahalla El-Kubra, Al Gharbiyah, Egypt; 11University of Florida, Gainesville, FL; 12Tucson Medical Center, Tucson, AZ; 13Liaquat University of Medical and Health Science, Jamshoro, Sindh, Pakistan
Introduction: Vedolizumab is a gut-selective, anti-integrin humanised monoclonal antibody that selectively targets α4β7 integrin, widely used for the treatment of inflammatory bowel diseases (IBD). The treatment response is variable, and the role of therapeutic drug monitoring (TDM) in optimising vedolizumab therapy remains unclear. This study aims to evaluate the relationship between vedolizumab trough concentrations (VTC) and clinical, endoscopic, and histologic outcomes in patients with IBD to guide the use of TDM in clinical practice.
Methods: A systematic search of PubMed/MEDLINE, Embase and Scopus was performed applying Boolean operators and MeSH keywords. PRISMA standards were followed, and the study was pre-registered on PROSPERO. Two authors independently screened 1,066 records after removing duplicates. 12 studies were included based on the inclusion criteria. Primary outcomes were clinical, endoscopic, and biologic remission; secondary outcomes were mucosal healing, steroid-free remission, response to dose optimisation, and adverse events. Risk of bias was evaluated using the NIH tool. Synthesising the data used Review Manager (RevMan v5.4.1), and pooled proportions with 95% confidence intervals were determined using a random-effects model.
Results: A total of 12 observational studies involving 3,259 patients with IBD (Crohn's disease and ulcerative colitis) were included. In the pooled proportion, clinical remission was 44% (95% CI: 13–80%), endoscopic remission 33% (95% CI: 23–43%), and steroid-free remission 39% (95% CI: 24–56%). Mucosal healing was seen in 37% of the patients (95% CI: 28–47%). The mean VTC was 18.76 μg/mL (95% CI: 12.18–25.34). The pooled response rate was 48% (95% CI: 30–67%) in patients who received dose optimisation. Serious adverse events were rare, with an overall occurrence of 8% (95% CI: 2–22%). Heterogeneity was extreme (I² = 95%), largely due to an outlier study, which was supported by sensitivity analyses and Baujat.
Discussion: This study implies a strong relationship between greater VTC and better clinical, endoscopic, and histologic results in IBD. Pooled data shows that VTC ≥18–24 μg/mL during induction and ≥12 μg/mL during maintenance are associated with remission. Optimal dosing resulted in ~50% response. Vedolizumab was very safe, with low immunogenicity and very few serious adverse events. The findings from the study support VTC-guided dosing in practice.

Figure: Forest Plots:
Figure 1. Forest Plot of Vedolizumab Trough Levels.
Figure 2. Forest Plot of Clinical Remission.
Figure 3. Forest Plot of Endoscopic Remission.
Figure 4. Forest Plot of Steroid Free Remission.

Figure: Forest Plots:
Figure 1. Forest Plot of Response to Dose Optimisation.
Figure 2. Forest Plot of Mucosal Healing.
Figure 3. Forest Plot of Any Adverse Event.
Figure 4. Forest Plot of Serious Adverse Events.
Disclosures:
K. Sai Varun Reddy indicated no relevant financial relationships.
Shree Rath indicated no relevant financial relationships.
Ammarah Tariq indicated no relevant financial relationships.
Muhammad Uzair indicated no relevant financial relationships.
Yousef M. Husseiny indicated no relevant financial relationships.
Anika Goel indicated no relevant financial relationships.
Arun Maloth indicated no relevant financial relationships.
Ayesha Arshad indicated no relevant financial relationships.
Noma Nazir indicated no relevant financial relationships.
Mohamed Abohalawa indicated no relevant financial relationships.
Adnan Bhat indicated no relevant financial relationships.
Fatima Aslam indicated no relevant financial relationships.
Ammara Tahir indicated no relevant financial relationships.
K. Sai Varun Reddy, 1, Shree Rath, MBBS2, Ammarah Tariq, MD3, Muhammad Uzair, MBBS4, Yousef M. Husseiny, MBBCh5, Anika Goel, 6, Arun Kumar. Maloth, MBBS7, Ayesha Arshad, MBBS8, Noma Nazir, MBBS9, Mohamed N. Abohalawa, 10, Adnan Bhat, MD11, Fatima Aslam, MBBS, MD12, Ammara Tahir, MBBS13. P3161 - Association Between Vedolizumab Trough Concentration and Clinical Outcomes in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
