Monday Poster Session
Category: IBD
P3158 - Risankizumab Effectiveness and Safety in Ulcerative Colitis: Real-World Data From a Large Tertiary Center
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Russell Yanofsky, MD (he/him/his)
University of Chicago Medicine, Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
Russell Yanofsky, MD1, Zachary D. Fine, BA2, Evan Fear, BSc1, Asher Shafrir, MD1, David Choi, PharmD3, Tenzin Choden, MD1, Sushila Dalal, MD1, Noa Krugliak Cleveland, MD2, Benjamin McDonald, MD, PhD2, Joel Pekow, MD2, Russell D. Cohen, MD4, David T. Rubin, MD5
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago, Chicago, IL; 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 4University of Chicago Medicine, Chicago, IL; 5University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Risankizumab (RZB) is an interleukin-23p19 inhibitor that was approved by the U.S. Food and Drug Administration in June 2024 for the treatment of moderately to severely active ulcerative colitis (UC). We report the real-world experience with RZB in UC in a large tertiary inflammatory bowel disease (IBD) center.
Methods: This is a cohort study in which we prospectively recruited patients with UC at the UChicago IBD Center who were treated with RZB at standard labeled intravenous loading and maintenance dosing. We performed clinical assessments at baseline, weeks 2, 4, 8, 12, and 26, using the Simplified Clinical Colitis Activity Index (SCCAI) and fecal calprotectin (FCP). Clinical remission was defined as SCCAI ≤2 and/or FCP≤150µg/g. Steroid-free remission was defined as SCCAI≤2 in the absence of steroid use. Adverse events were documented. Results were stratified based on prior exposure to ustekinumab (UST). Statistical analyses were performed using R v4.1.3.
Results: 49 patients were recruited (Table 1), with 44 (90.0%) patients previously exposed to advanced therapies and 18 (36.7%) previously exposed to UST. Median SCCAI throughout follow-up are shown in Figure 1. Baseline median (interquartile range [IQR]) SCCAI and FCP were 3.0 (1.0-5.8) and 476.0 μg/g (105.2-1156.2), respectively. At week 8, median (IQR) SCCAI was 1.0 (0.0-2.0), with 31/41 (75.6%) in clinical remission and 29/41 (70.7%) in steroid-free remission. At week 26, the median (IQR) SCCAI was 1.0 (0.0-1.0), with 22/25 (88.0%) in clinical remission and 20/25 (80.0%) in steroid-free remission. Median (IQR) FCP over the study period (n=29) was 109 μg/g (60-645), with 16/29 (55.2%) in biomarker remission, and similar in patients exposed to UST (n=9, 79.9 μg/g [33.0-382.0]) and not exposed (n=20, 114.5 μg/g [69.8-736.2]). Two (4.1%) patients were hospitalized for severe UC and no patients underwent colectomy. 4 (8.2%) patients stopped RZB due to clinically active disease, and one (2.0%) patient stopped therapy due to a diffuse rash. Other adverse events included fatigue (n=7, 14.3%), headache (n=1, 2.0%), constipation (n=1, 2.0%), and low-grade fever with the first infusion (n=1, 2.0%).
Discussion: We present the largest real-world study of the effectiveness and safety of RZB in patients with UC, including patients previously exposed to UST. In this interim analysis, RZB is an effective and safe therapy for patients with moderately to severely active UC. Longer-term follow-up is ongoing.
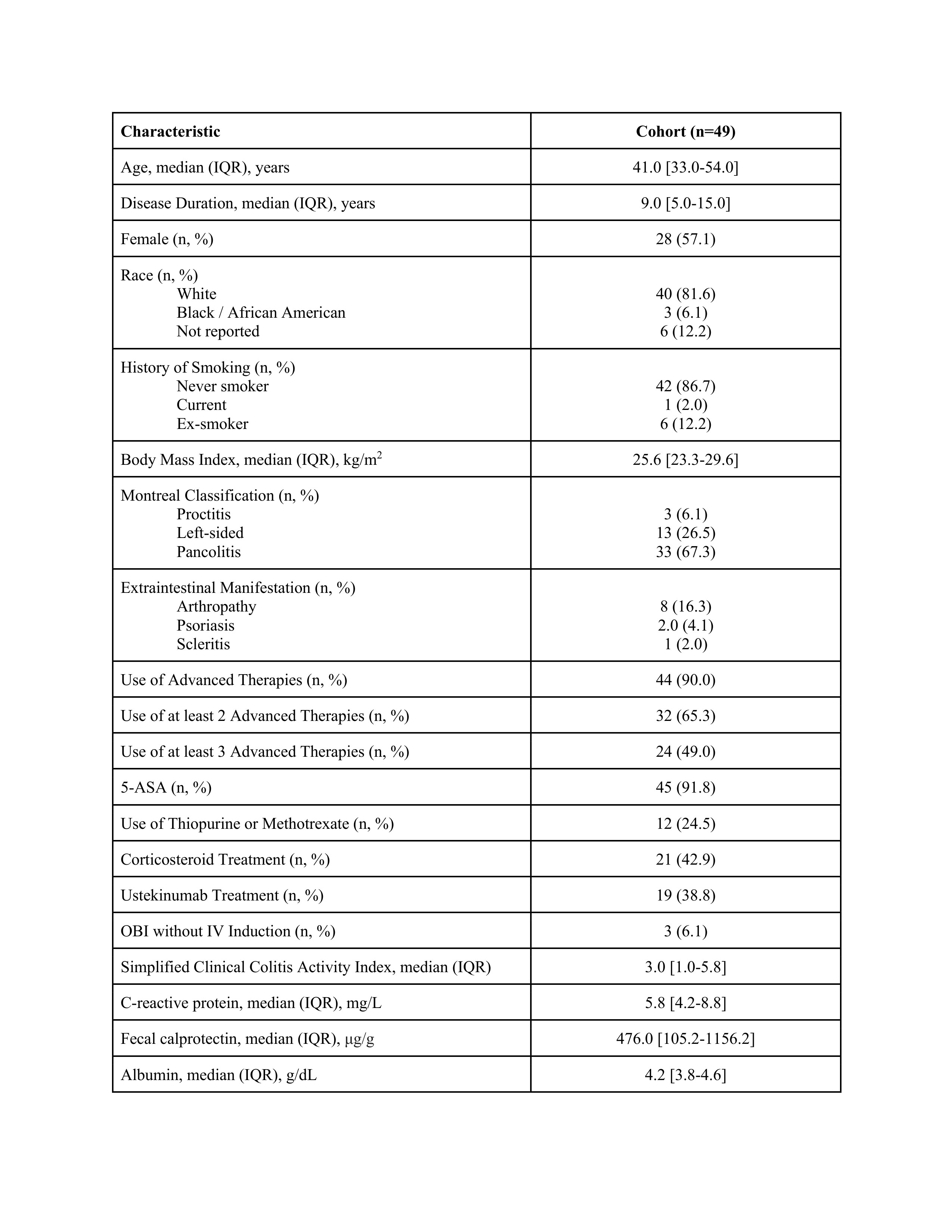
Figure: Table 1. Baseline characteristics of study cohort. Abbreviations: IQR = interquartile range. N = number. BMI = body mass index. IV = intravenous. OBI = on-body injector. IV = intravenous. 5-ASA = 5-aminosalicylic acid.

Figure: Figure 1. Simplified Clinical Colitis Activity Index Scores for All Patients at Week 0, 2, 4, 8, 12, and 26
Disclosures:
Russell Yanofsky indicated no relevant financial relationships.
Zachary Fine indicated no relevant financial relationships.
Evan Fear indicated no relevant financial relationships.
Asher Shafrir indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Tenzin Choden indicated no relevant financial relationships.
Sushila Dalal: Abbvie – Speakers Bureau.
Noa Krugliak Cleveland: Johnson & Johnson – Consultant. NueroLogica – Consultant.
Benjamin McDonald: Iterative Health – Consultant.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Russell Yanofsky, MD1, Zachary D. Fine, BA2, Evan Fear, BSc1, Asher Shafrir, MD1, David Choi, PharmD3, Tenzin Choden, MD1, Sushila Dalal, MD1, Noa Krugliak Cleveland, MD2, Benjamin McDonald, MD, PhD2, Joel Pekow, MD2, Russell D. Cohen, MD4, David T. Rubin, MD5. P3158 - Risankizumab Effectiveness and Safety in Ulcerative Colitis: Real-World Data From a Large Tertiary Center, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago, Chicago, IL; 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 4University of Chicago Medicine, Chicago, IL; 5University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Risankizumab (RZB) is an interleukin-23p19 inhibitor that was approved by the U.S. Food and Drug Administration in June 2024 for the treatment of moderately to severely active ulcerative colitis (UC). We report the real-world experience with RZB in UC in a large tertiary inflammatory bowel disease (IBD) center.
Methods: This is a cohort study in which we prospectively recruited patients with UC at the UChicago IBD Center who were treated with RZB at standard labeled intravenous loading and maintenance dosing. We performed clinical assessments at baseline, weeks 2, 4, 8, 12, and 26, using the Simplified Clinical Colitis Activity Index (SCCAI) and fecal calprotectin (FCP). Clinical remission was defined as SCCAI ≤2 and/or FCP≤150µg/g. Steroid-free remission was defined as SCCAI≤2 in the absence of steroid use. Adverse events were documented. Results were stratified based on prior exposure to ustekinumab (UST). Statistical analyses were performed using R v4.1.3.
Results: 49 patients were recruited (Table 1), with 44 (90.0%) patients previously exposed to advanced therapies and 18 (36.7%) previously exposed to UST. Median SCCAI throughout follow-up are shown in Figure 1. Baseline median (interquartile range [IQR]) SCCAI and FCP were 3.0 (1.0-5.8) and 476.0 μg/g (105.2-1156.2), respectively. At week 8, median (IQR) SCCAI was 1.0 (0.0-2.0), with 31/41 (75.6%) in clinical remission and 29/41 (70.7%) in steroid-free remission. At week 26, the median (IQR) SCCAI was 1.0 (0.0-1.0), with 22/25 (88.0%) in clinical remission and 20/25 (80.0%) in steroid-free remission. Median (IQR) FCP over the study period (n=29) was 109 μg/g (60-645), with 16/29 (55.2%) in biomarker remission, and similar in patients exposed to UST (n=9, 79.9 μg/g [33.0-382.0]) and not exposed (n=20, 114.5 μg/g [69.8-736.2]). Two (4.1%) patients were hospitalized for severe UC and no patients underwent colectomy. 4 (8.2%) patients stopped RZB due to clinically active disease, and one (2.0%) patient stopped therapy due to a diffuse rash. Other adverse events included fatigue (n=7, 14.3%), headache (n=1, 2.0%), constipation (n=1, 2.0%), and low-grade fever with the first infusion (n=1, 2.0%).
Discussion: We present the largest real-world study of the effectiveness and safety of RZB in patients with UC, including patients previously exposed to UST. In this interim analysis, RZB is an effective and safe therapy for patients with moderately to severely active UC. Longer-term follow-up is ongoing.

Figure: Table 1. Baseline characteristics of study cohort. Abbreviations: IQR = interquartile range. N = number. BMI = body mass index. IV = intravenous. OBI = on-body injector. IV = intravenous. 5-ASA = 5-aminosalicylic acid.

Figure: Figure 1. Simplified Clinical Colitis Activity Index Scores for All Patients at Week 0, 2, 4, 8, 12, and 26
Disclosures:
Russell Yanofsky indicated no relevant financial relationships.
Zachary Fine indicated no relevant financial relationships.
Evan Fear indicated no relevant financial relationships.
Asher Shafrir indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Tenzin Choden indicated no relevant financial relationships.
Sushila Dalal: Abbvie – Speakers Bureau.
Noa Krugliak Cleveland: Johnson & Johnson – Consultant. NueroLogica – Consultant.
Benjamin McDonald: Iterative Health – Consultant.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Russell Yanofsky, MD1, Zachary D. Fine, BA2, Evan Fear, BSc1, Asher Shafrir, MD1, David Choi, PharmD3, Tenzin Choden, MD1, Sushila Dalal, MD1, Noa Krugliak Cleveland, MD2, Benjamin McDonald, MD, PhD2, Joel Pekow, MD2, Russell D. Cohen, MD4, David T. Rubin, MD5. P3158 - Risankizumab Effectiveness and Safety in Ulcerative Colitis: Real-World Data From a Large Tertiary Center, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
