Monday Poster Session
Category: GI Bleeding
P3098 - From Targeted Therapy to Targeted Injury: Osimertinib-Associated Gastric Ulcer Bleeding
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aakash Aakash, MD (he/him/his)
Florida State University
Cape Coral, FL
Presenting Author(s)
Fnu Aakash, MD1, Sunny Kumar, MD2, Osama Abdur Rehman, MD1, FNU Payal, MD3, Aasta Kumari, MD4, Vivek Singh, MBBS5, Deepak Kumar, MBBS, MD6, Abdus Sameey Anwar, MBBS7, Silpa Choday, MD8, Sri Harsha Boppana, MBBS, MD9, Gautam Maddineni, MD1, Binay Panjiyar, MD10
1Florida State University, Cape Coral, FL; 2Wright Center for Graduate Medical Education, Scranton, PA; 3Monmouth Medical Center, Robert Wood Johnson Medical School of Rutgers University, Long Branch, NJ; 4North Central Bronx Hospital, New York, NY; 5FLORIDA STATE UNIVERSITY, Cape Coral, FL; 6Northwell Health, Port Jefferson, NY; 7College of Medical Sciences, Bharatpur, Dhanbad, Jharkhand, India; 8Creighton University School of Medicine, Phoenix, AZ; 9Nassau University Medical Center, East Meadow, NY; 10NorthShore University Hospital, Manhasset, NY
Introduction: Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), is widely used as first-line therapy in non-small-cell lung cancer (NSCLC) with EGFR mutations. While it is generally well-tolerated, common gastrointestinal (GI) adverse effects include diarrhea and mucositis. Severe GI bleeding is exceedingly rare and has not been previously reported. We describe the first known case of a gastric ulcer with upper GI hemorrhage attributed to osimertinib.
Case Description/
Methods: A 57-year-old male non-smoker with Stage IV NSCLC harboring an EGFR exon 19 deletion was started on osimertinib following the identification of brain and bone metastases. Within one week of therapy initiation, he developed epigastric pain, melena, dyspnea, and fatigue. Laboratory studies revealed anemia, and examination noted pallor and epigastric tenderness. He denied NSAID or steroid use, alcohol consumption, or prior GI disease. H. pylori testing was negative. Upper endoscopy revealed a large prepyloric ulcer without signs of malignancy or infection. Given the acute onset after drug initiation, resolution of symptoms with discontinuation, and lack of alternative etiologies, a diagnosis of osimertinib-induced gastric ulceration was made. The patient improved with high-dose pantoprazole, and the follow-up endoscopy showed complete healing. He was transitioned to aumolertinib, which was well tolerated without recurrence.
Discussion: Several factors supported osimertinib as the causative agent: (1) rapid onset of symptoms within one week of starting therapy, (2) resolution of symptoms and mucosal healing upon drug cessation, (3) exclusion of other common causes of ulcer disease, and (4) the known biological mechanism by which EGFR inhibition impairs mucosal integrity and epithelial repair. Furthermore, the patient tolerated aumolertinib without recurrence, suggesting drug-specific rather than class-wide toxicity. Although EGFR-TKIs are generally associated with mild GI symptoms, this case highlights a rare but clinically significant risk of ulceration and bleeding. Clinicians should maintain a high index of suspicion for GI complications in patients presenting with early GI symptoms or anemia during osimertinib therapy. Prompt recognition, endoscopic evaluation, and cessation of the offending agent are key to preventing severe outcomes. Additional research is needed to characterize the mechanisms and incidence of GI mucosal injury with third-generation EGFR-TKIs.
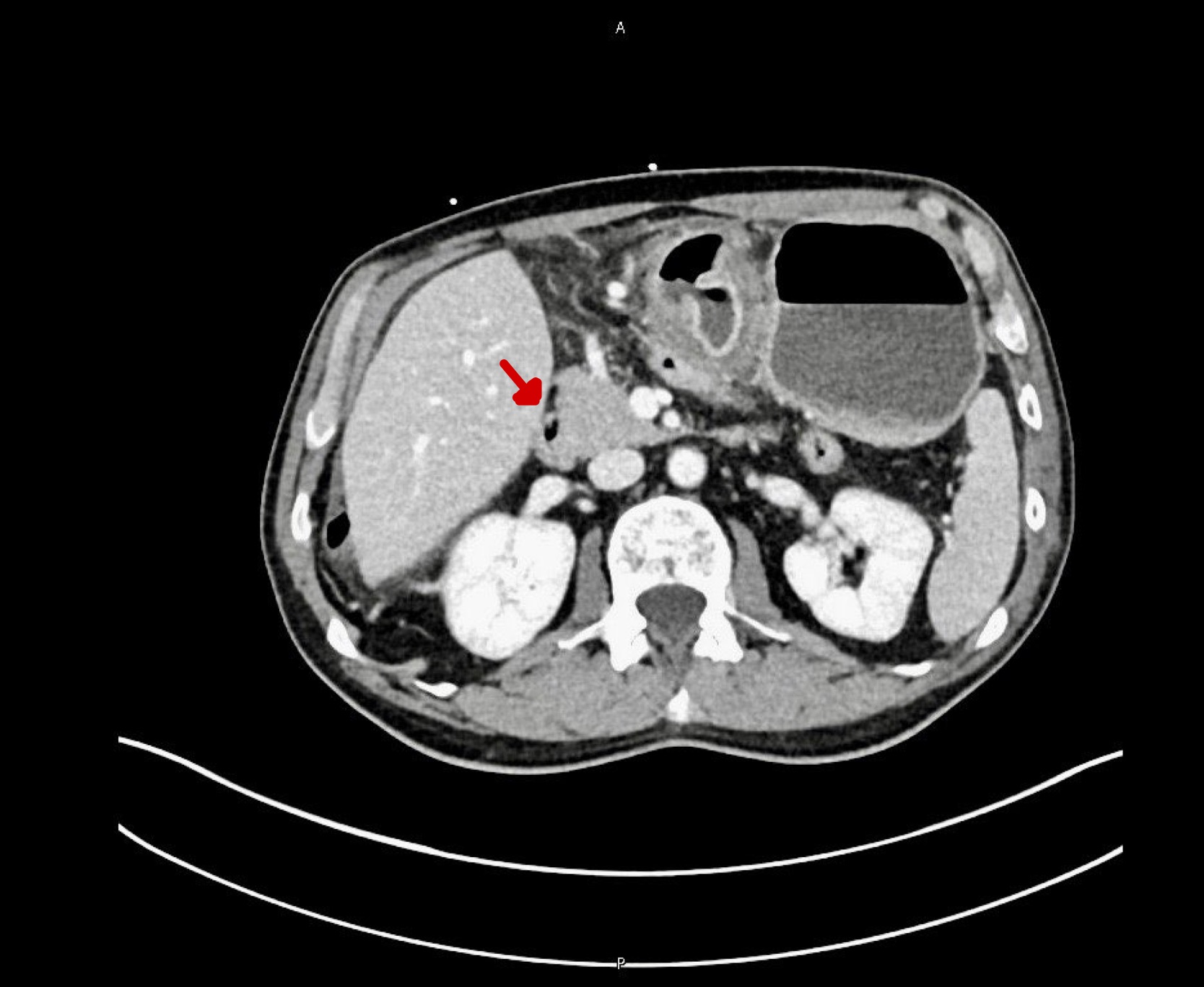
Figure: Figure 1: " Endoscopic view showing gastric bleeding"
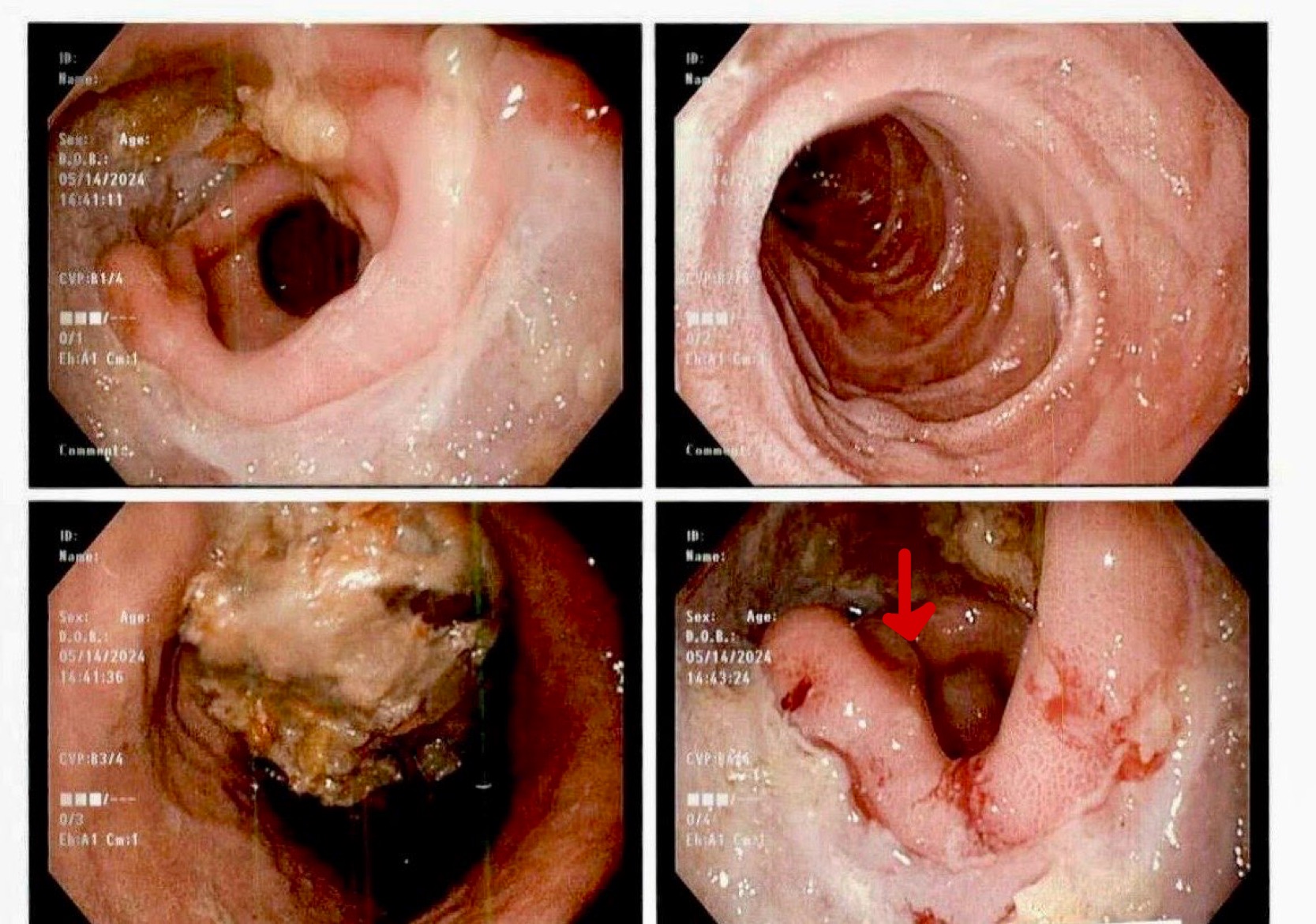
Figure: Figure 2: " CT scan image showing gastric bleeding"
Disclosures:
Fnu Aakash indicated no relevant financial relationships.
Sunny Kumar indicated no relevant financial relationships.
Osama Abdur Rehman indicated no relevant financial relationships.
FNU Payal indicated no relevant financial relationships.
Aasta Kumari indicated no relevant financial relationships.
Vivek Singh indicated no relevant financial relationships.
Deepak Kumar indicated no relevant financial relationships.
Abdus Sameey Anwar indicated no relevant financial relationships.
Silpa Choday indicated no relevant financial relationships.
Sri Harsha Boppana indicated no relevant financial relationships.
Gautam Maddineni indicated no relevant financial relationships.
Binay Panjiyar indicated no relevant financial relationships.
Fnu Aakash, MD1, Sunny Kumar, MD2, Osama Abdur Rehman, MD1, FNU Payal, MD3, Aasta Kumari, MD4, Vivek Singh, MBBS5, Deepak Kumar, MBBS, MD6, Abdus Sameey Anwar, MBBS7, Silpa Choday, MD8, Sri Harsha Boppana, MBBS, MD9, Gautam Maddineni, MD1, Binay Panjiyar, MD10. P3098 - From Targeted Therapy to Targeted Injury: Osimertinib-Associated Gastric Ulcer Bleeding, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Florida State University, Cape Coral, FL; 2Wright Center for Graduate Medical Education, Scranton, PA; 3Monmouth Medical Center, Robert Wood Johnson Medical School of Rutgers University, Long Branch, NJ; 4North Central Bronx Hospital, New York, NY; 5FLORIDA STATE UNIVERSITY, Cape Coral, FL; 6Northwell Health, Port Jefferson, NY; 7College of Medical Sciences, Bharatpur, Dhanbad, Jharkhand, India; 8Creighton University School of Medicine, Phoenix, AZ; 9Nassau University Medical Center, East Meadow, NY; 10NorthShore University Hospital, Manhasset, NY
Introduction: Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), is widely used as first-line therapy in non-small-cell lung cancer (NSCLC) with EGFR mutations. While it is generally well-tolerated, common gastrointestinal (GI) adverse effects include diarrhea and mucositis. Severe GI bleeding is exceedingly rare and has not been previously reported. We describe the first known case of a gastric ulcer with upper GI hemorrhage attributed to osimertinib.
Case Description/
Methods: A 57-year-old male non-smoker with Stage IV NSCLC harboring an EGFR exon 19 deletion was started on osimertinib following the identification of brain and bone metastases. Within one week of therapy initiation, he developed epigastric pain, melena, dyspnea, and fatigue. Laboratory studies revealed anemia, and examination noted pallor and epigastric tenderness. He denied NSAID or steroid use, alcohol consumption, or prior GI disease. H. pylori testing was negative. Upper endoscopy revealed a large prepyloric ulcer without signs of malignancy or infection. Given the acute onset after drug initiation, resolution of symptoms with discontinuation, and lack of alternative etiologies, a diagnosis of osimertinib-induced gastric ulceration was made. The patient improved with high-dose pantoprazole, and the follow-up endoscopy showed complete healing. He was transitioned to aumolertinib, which was well tolerated without recurrence.
Discussion: Several factors supported osimertinib as the causative agent: (1) rapid onset of symptoms within one week of starting therapy, (2) resolution of symptoms and mucosal healing upon drug cessation, (3) exclusion of other common causes of ulcer disease, and (4) the known biological mechanism by which EGFR inhibition impairs mucosal integrity and epithelial repair. Furthermore, the patient tolerated aumolertinib without recurrence, suggesting drug-specific rather than class-wide toxicity. Although EGFR-TKIs are generally associated with mild GI symptoms, this case highlights a rare but clinically significant risk of ulceration and bleeding. Clinicians should maintain a high index of suspicion for GI complications in patients presenting with early GI symptoms or anemia during osimertinib therapy. Prompt recognition, endoscopic evaluation, and cessation of the offending agent are key to preventing severe outcomes. Additional research is needed to characterize the mechanisms and incidence of GI mucosal injury with third-generation EGFR-TKIs.

Figure: Figure 1: " Endoscopic view showing gastric bleeding"

Figure: Figure 2: " CT scan image showing gastric bleeding"
Disclosures:
Fnu Aakash indicated no relevant financial relationships.
Sunny Kumar indicated no relevant financial relationships.
Osama Abdur Rehman indicated no relevant financial relationships.
FNU Payal indicated no relevant financial relationships.
Aasta Kumari indicated no relevant financial relationships.
Vivek Singh indicated no relevant financial relationships.
Deepak Kumar indicated no relevant financial relationships.
Abdus Sameey Anwar indicated no relevant financial relationships.
Silpa Choday indicated no relevant financial relationships.
Sri Harsha Boppana indicated no relevant financial relationships.
Gautam Maddineni indicated no relevant financial relationships.
Binay Panjiyar indicated no relevant financial relationships.
Fnu Aakash, MD1, Sunny Kumar, MD2, Osama Abdur Rehman, MD1, FNU Payal, MD3, Aasta Kumari, MD4, Vivek Singh, MBBS5, Deepak Kumar, MBBS, MD6, Abdus Sameey Anwar, MBBS7, Silpa Choday, MD8, Sri Harsha Boppana, MBBS, MD9, Gautam Maddineni, MD1, Binay Panjiyar, MD10. P3098 - From Targeted Therapy to Targeted Injury: Osimertinib-Associated Gastric Ulcer Bleeding, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
