Monday Poster Session
Category: Functional Bowel Disease
P2952 - Limited Diagnostic Concordance Between ARFID Screenings and Provider Documentation in a Neurogastroenterology Population
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MB
Madeline Berschback, MD (she/her/hers)
Massachusetts General Hospital
Boston, MA
Presenting Author(s)
Madeline Berschback, MD1, Helen Burton-Murray, PhD2, Rebecca Karlson, BS1, Sophie R. Abber, MS3, Bianca C. Zussino, BSc1, Jenna Clukey, MS1, Benjamin Wasserman, 1, Kyle Staller, MD, MPH1
1Massachusetts General Hospital, Boston, MA; 2Massachusetts General Hospital, Harvard Medical School, Boston, MA; 3Massachusetts General Hospital, San Diego, CA
Introduction: Avoidant/restrictive food intake disorder (ARFID) is characterized by limited food intake not driven by body image concerns. The Nine Item ARFID Screen (NIAS) has validated screening cutoffs in an eating disorder population, but their generalizability and clinical utility in neurogastroenterology is unknown. We tested criterion-related concurrent validity of the proposed NIAS cutoffs to effectively identify clinically significant ARFID among patients presenting to an adult neurogastroenterology clinic.
Methods: We conducted a retrospective chart review of consecutive adult neurogastroenterology consultation visits from April 2023 to December 2024 at a large tertiary care center. Prior to their visit, patients completed a battery of surveys as part of routine care, including the NIAS. Positive ARFID screen was determined by proposed NIAS cutoffs (Picky≥10, Appetite≥9, and/or Fear≥10). Trained coders reviewed consult notes to determine whether ARFID was documented by the provider and we tested concordance between NIAS and ARFID documentation using logistic regression.
Results: Of 241 patients who completed the NIAS, 14 (5.8%) were identified as having provider-documented ARFID by chart review. There was no significant difference in age, gender identity, sex, race, ethnicity, or BMI between the ARFID and non-ARFID groups (Table 1). Overall, 39% of patients screened positive for ARFID on at least one NIAS subscale: 25.7% for Appetite, 24.1% for Fear, and 18.3% for Picky Eating. Screening positively on the NIAS yielded a non-significant association with chart-confirmed ARFID (OR 2.2, Z=1.4, p=0.16, 95% CI: 0.7-6.5) in univariable logistic regression. NIAS cutoffs correctly classified n=8 (57.1%) of chart-confirmed ARFID, but incorrectly classified n=86 (37.9%) without chart-confirmed ARFID (Figure 1).
Discussion: Among patients presenting for initial consultation to a neurogastroenterology clinic, NIAS-positive ARFID screens showed limited concordance with clinician-determined diagnoses. There is reasonable concern among GI providers that existing screening tools could overinflate the prevalence of ARFID in neurogastroenterology populations. Further research is needed to identify the utility of the NIAS to screen for ARFID in a neurogastroenterology setting, possibly with the identification of alternative cutoffs.
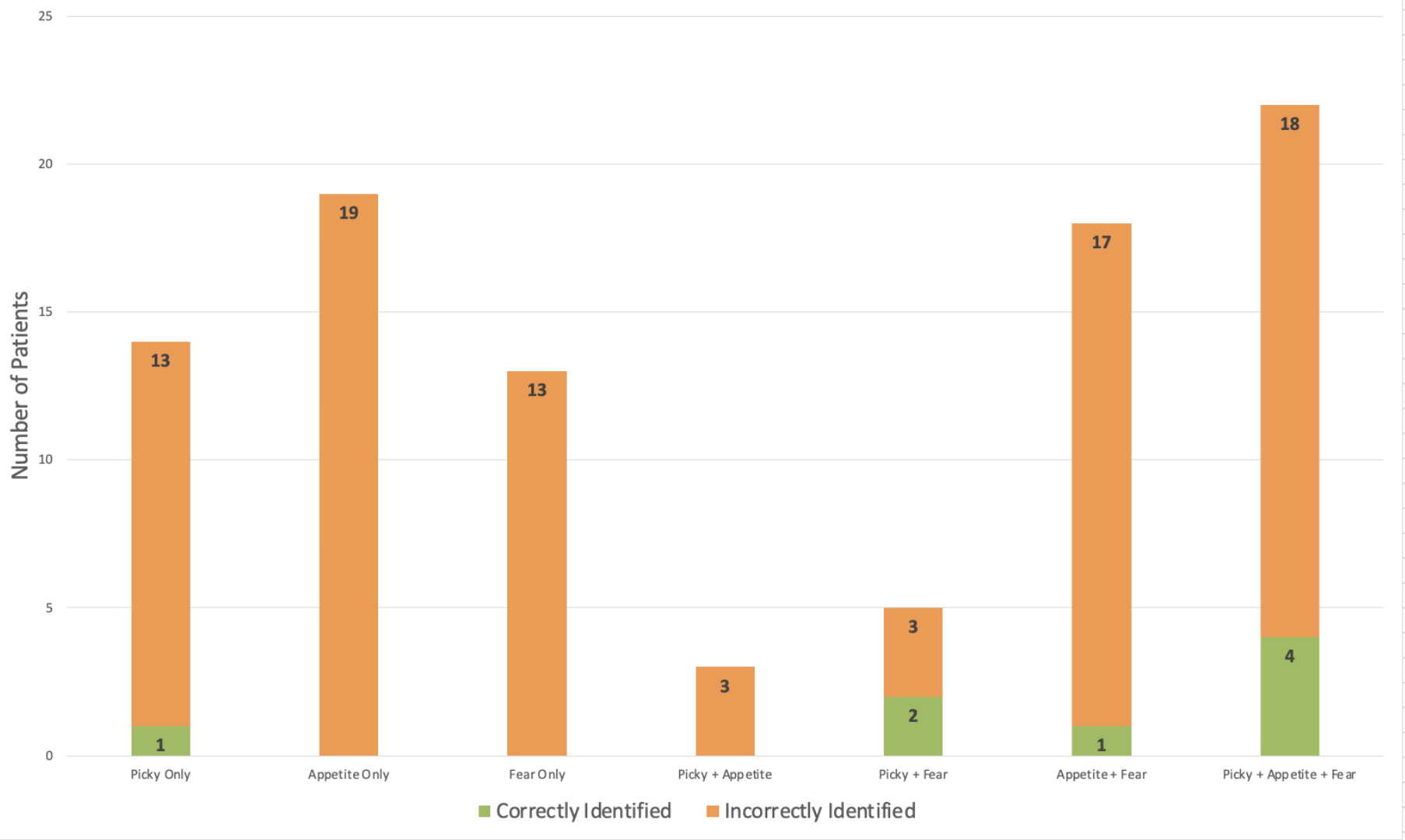
Figure: Baseline Characteristics of Patients by ARFID Status
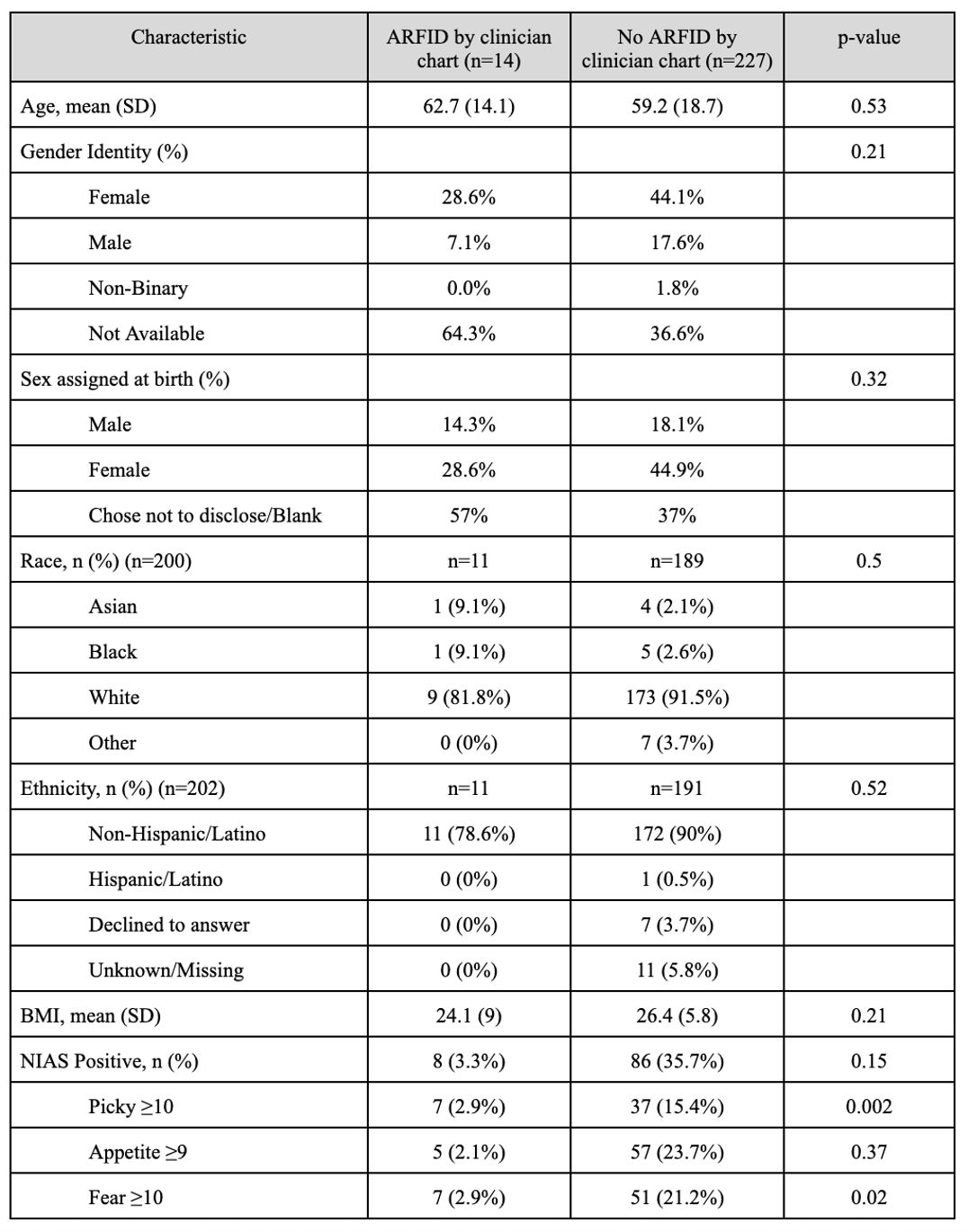
Figure: Correct and Incorrect ARFID Identification Across NIAS Subscales
Disclosures:
Madeline Berschback indicated no relevant financial relationships.
Helen Burton-Murray indicated no relevant financial relationships.
Rebecca Karlson indicated no relevant financial relationships.
Sophie Abber indicated no relevant financial relationships.
Bianca Zussino indicated no relevant financial relationships.
Jenna Clukey indicated no relevant financial relationships.
Benjamin Wasserman indicated no relevant financial relationships.
Kyle Staller: Ardelyx – Grant/Research Support. Gemelli – Consultant. Laborie – Consultant. Mahana – Consultant. Salix – Consultant. Takeda – Expert witness.
Madeline Berschback, MD1, Helen Burton-Murray, PhD2, Rebecca Karlson, BS1, Sophie R. Abber, MS3, Bianca C. Zussino, BSc1, Jenna Clukey, MS1, Benjamin Wasserman, 1, Kyle Staller, MD, MPH1. P2952 - Limited Diagnostic Concordance Between ARFID Screenings and Provider Documentation in a Neurogastroenterology Population, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Massachusetts General Hospital, Boston, MA; 2Massachusetts General Hospital, Harvard Medical School, Boston, MA; 3Massachusetts General Hospital, San Diego, CA
Introduction: Avoidant/restrictive food intake disorder (ARFID) is characterized by limited food intake not driven by body image concerns. The Nine Item ARFID Screen (NIAS) has validated screening cutoffs in an eating disorder population, but their generalizability and clinical utility in neurogastroenterology is unknown. We tested criterion-related concurrent validity of the proposed NIAS cutoffs to effectively identify clinically significant ARFID among patients presenting to an adult neurogastroenterology clinic.
Methods: We conducted a retrospective chart review of consecutive adult neurogastroenterology consultation visits from April 2023 to December 2024 at a large tertiary care center. Prior to their visit, patients completed a battery of surveys as part of routine care, including the NIAS. Positive ARFID screen was determined by proposed NIAS cutoffs (Picky≥10, Appetite≥9, and/or Fear≥10). Trained coders reviewed consult notes to determine whether ARFID was documented by the provider and we tested concordance between NIAS and ARFID documentation using logistic regression.
Results: Of 241 patients who completed the NIAS, 14 (5.8%) were identified as having provider-documented ARFID by chart review. There was no significant difference in age, gender identity, sex, race, ethnicity, or BMI between the ARFID and non-ARFID groups (Table 1). Overall, 39% of patients screened positive for ARFID on at least one NIAS subscale: 25.7% for Appetite, 24.1% for Fear, and 18.3% for Picky Eating. Screening positively on the NIAS yielded a non-significant association with chart-confirmed ARFID (OR 2.2, Z=1.4, p=0.16, 95% CI: 0.7-6.5) in univariable logistic regression. NIAS cutoffs correctly classified n=8 (57.1%) of chart-confirmed ARFID, but incorrectly classified n=86 (37.9%) without chart-confirmed ARFID (Figure 1).
Discussion: Among patients presenting for initial consultation to a neurogastroenterology clinic, NIAS-positive ARFID screens showed limited concordance with clinician-determined diagnoses. There is reasonable concern among GI providers that existing screening tools could overinflate the prevalence of ARFID in neurogastroenterology populations. Further research is needed to identify the utility of the NIAS to screen for ARFID in a neurogastroenterology setting, possibly with the identification of alternative cutoffs.

Figure: Baseline Characteristics of Patients by ARFID Status

Figure: Correct and Incorrect ARFID Identification Across NIAS Subscales
Disclosures:
Madeline Berschback indicated no relevant financial relationships.
Helen Burton-Murray indicated no relevant financial relationships.
Rebecca Karlson indicated no relevant financial relationships.
Sophie Abber indicated no relevant financial relationships.
Bianca Zussino indicated no relevant financial relationships.
Jenna Clukey indicated no relevant financial relationships.
Benjamin Wasserman indicated no relevant financial relationships.
Kyle Staller: Ardelyx – Grant/Research Support. Gemelli – Consultant. Laborie – Consultant. Mahana – Consultant. Salix – Consultant. Takeda – Expert witness.
Madeline Berschback, MD1, Helen Burton-Murray, PhD2, Rebecca Karlson, BS1, Sophie R. Abber, MS3, Bianca C. Zussino, BSc1, Jenna Clukey, MS1, Benjamin Wasserman, 1, Kyle Staller, MD, MPH1. P2952 - Limited Diagnostic Concordance Between ARFID Screenings and Provider Documentation in a Neurogastroenterology Population, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

