Monday Poster Session
Category: Esophagus
P2760 - Development of a Patient Reported Outcome Measure for Barrett’s Esophagus (PROBE): Concept Elicitation and Item Generation
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- SE
Swathi Eluri, MD, MSCR
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Award: ACG Presidential Poster Award
Swathi Eluri, MD, MSCR1, Jason Egginton, MPH2, Prasad Iyer, MD, MS3, Nicholas J. Shaheen, MD, MPH, MACG4, Laurie Keefer, PhD, FACG5
1Mayo Clinic, Jacksonville, FL; 2Mayo Clinic, Rochester, MN; 3Mayo Clinic, Phoenix, AZ; 4University of North Carolina at Chapel Hill, Chapel Hill, NC; 5Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Barrett’s esophagus (BE) lacks a disease-specific patient-reported outcome (PRO) instrument to assess health-related quality of life (HRQOL) to capture the patient voice and serve as an outcome in clinical research. Therefore, we aimed to develop a BE-specific PRO instrument (PROBE).
Methods: We conducted semi-structured concept elicitation interviews with 36 patients stratified by management stage: surveillance (n=13), active endoscopic eradication therapy (EET) (n=11), and post-complete eradication of intestinal metaplasia (CEIM) surveillance (n=12). Interviews were transcribed and analyzed using a Framework approach to generate codes and themes. Based on identified concepts, a preliminary item pool was drafted, refined iteratively for clarity and content validity, and grouped into conceptual domains to reflect HRQOL in BE.
Results: Patients (mean age 65.9 ± 9.0 years, 66.7% male, 56% with smoking history) reported baseline histologies: NDBE (30.6%), IND (11.1%), LGD (16.7%), HGD (30.6%), and IMC (11.1%). GERD symptom frequency on medications varied: none (47.2%), monthly (27.8%), and weekly/daily (25%).
Surveillance Phase: Patients described long-term management burdens, altered dietary and sleep habits, and persistent anxiety about cancer risk and PPI side effects. Some reported logistical/financial challenges and medication costs (Figure).
Active EET Phase: Patients recalled procedural pain, post-treatment dietary limitations, and lifestyle changes to minimize symptoms. Emotional impacts included fear of cancer and concerns about long-term medication use. Some had access issues.
Post-CEIM Surveillance Phase: Patients reported lasting discomfort from ablation or dilation, dietary restrictions, concerns about recurrence, financial concerns, and a need for clearer post-treatment guidance.
From these insights, 40 candidate items were drafted and categorized into 7 domains: physical symptoms, procedural impact, lifestyle modification, emotional well-being, medication concerns, healthcare access/logistics, and disease understanding. 25-items (Table) were chosen for evaluation in a patient validation cohort sample.
Discussion: Themes of physical discomfort, lifestyle disruption, emotional distress, and care-related burdens across all treatment stages were common and informed the development of a patient-centered item pool. Future steps include psychometric validation, and finalization of the BE-PRO instrument for use in clinical and research settings.
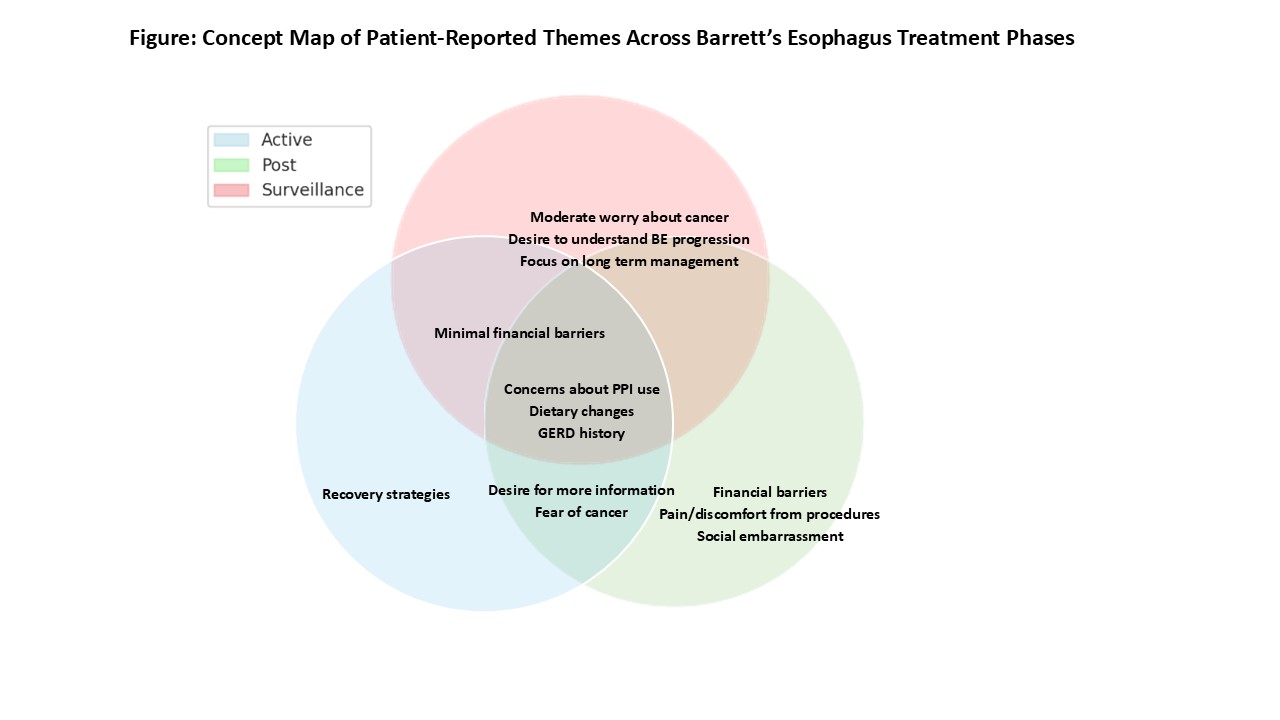
Figure: Figure: Concept Map of Patient-Reported Themes Across Barrett’s Esophagus Treatment Phases

Figure: Table: Preliminary list of 25 candidate items for measuring quality of life (QOL) in patients with Barrett’s esophagus (BE) across 7 distinct domains
Disclosures:
Swathi Eluri indicated no relevant financial relationships.
Jason Egginton indicated no relevant financial relationships.
Prasad Iyer: CDx medical – Consultant, Grant/Research Support. Exact – Grant/Research Support. Exact Sciences – Consultant, Grant/Research Support. Medtronic – Consultant. Pentax Medical – Consultant, Grant/Research Support.
Nicholas Shaheen: Aqua – Grant/Research Support. CDx – Grant/Research Support. Cook Medical – Consultant. GIE Medical – Grant/Research Support. Interpace Diagnostics – Grant/Research Support. Lucid Diagnostics – Grant/Research Support. Medtronic – Grant/Research Support. Pentax – Grant/Research Support. Steris – Grant/Research Support.
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Swathi Eluri, MD, MSCR1, Jason Egginton, MPH2, Prasad Iyer, MD, MS3, Nicholas J. Shaheen, MD, MPH, MACG4, Laurie Keefer, PhD, FACG5. P2760 - Development of a Patient Reported Outcome Measure for Barrett’s Esophagus (PROBE): Concept Elicitation and Item Generation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Swathi Eluri, MD, MSCR1, Jason Egginton, MPH2, Prasad Iyer, MD, MS3, Nicholas J. Shaheen, MD, MPH, MACG4, Laurie Keefer, PhD, FACG5
1Mayo Clinic, Jacksonville, FL; 2Mayo Clinic, Rochester, MN; 3Mayo Clinic, Phoenix, AZ; 4University of North Carolina at Chapel Hill, Chapel Hill, NC; 5Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Barrett’s esophagus (BE) lacks a disease-specific patient-reported outcome (PRO) instrument to assess health-related quality of life (HRQOL) to capture the patient voice and serve as an outcome in clinical research. Therefore, we aimed to develop a BE-specific PRO instrument (PROBE).
Methods: We conducted semi-structured concept elicitation interviews with 36 patients stratified by management stage: surveillance (n=13), active endoscopic eradication therapy (EET) (n=11), and post-complete eradication of intestinal metaplasia (CEIM) surveillance (n=12). Interviews were transcribed and analyzed using a Framework approach to generate codes and themes. Based on identified concepts, a preliminary item pool was drafted, refined iteratively for clarity and content validity, and grouped into conceptual domains to reflect HRQOL in BE.
Results: Patients (mean age 65.9 ± 9.0 years, 66.7% male, 56% with smoking history) reported baseline histologies: NDBE (30.6%), IND (11.1%), LGD (16.7%), HGD (30.6%), and IMC (11.1%). GERD symptom frequency on medications varied: none (47.2%), monthly (27.8%), and weekly/daily (25%).
Surveillance Phase: Patients described long-term management burdens, altered dietary and sleep habits, and persistent anxiety about cancer risk and PPI side effects. Some reported logistical/financial challenges and medication costs (Figure).
Active EET Phase: Patients recalled procedural pain, post-treatment dietary limitations, and lifestyle changes to minimize symptoms. Emotional impacts included fear of cancer and concerns about long-term medication use. Some had access issues.
Post-CEIM Surveillance Phase: Patients reported lasting discomfort from ablation or dilation, dietary restrictions, concerns about recurrence, financial concerns, and a need for clearer post-treatment guidance.
From these insights, 40 candidate items were drafted and categorized into 7 domains: physical symptoms, procedural impact, lifestyle modification, emotional well-being, medication concerns, healthcare access/logistics, and disease understanding. 25-items (Table) were chosen for evaluation in a patient validation cohort sample.
Discussion: Themes of physical discomfort, lifestyle disruption, emotional distress, and care-related burdens across all treatment stages were common and informed the development of a patient-centered item pool. Future steps include psychometric validation, and finalization of the BE-PRO instrument for use in clinical and research settings.

Figure: Figure: Concept Map of Patient-Reported Themes Across Barrett’s Esophagus Treatment Phases

Figure: Table: Preliminary list of 25 candidate items for measuring quality of life (QOL) in patients with Barrett’s esophagus (BE) across 7 distinct domains
Disclosures:
Swathi Eluri indicated no relevant financial relationships.
Jason Egginton indicated no relevant financial relationships.
Prasad Iyer: CDx medical – Consultant, Grant/Research Support. Exact – Grant/Research Support. Exact Sciences – Consultant, Grant/Research Support. Medtronic – Consultant. Pentax Medical – Consultant, Grant/Research Support.
Nicholas Shaheen: Aqua – Grant/Research Support. CDx – Grant/Research Support. Cook Medical – Consultant. GIE Medical – Grant/Research Support. Interpace Diagnostics – Grant/Research Support. Lucid Diagnostics – Grant/Research Support. Medtronic – Grant/Research Support. Pentax – Grant/Research Support. Steris – Grant/Research Support.
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Swathi Eluri, MD, MSCR1, Jason Egginton, MPH2, Prasad Iyer, MD, MS3, Nicholas J. Shaheen, MD, MPH, MACG4, Laurie Keefer, PhD, FACG5. P2760 - Development of a Patient Reported Outcome Measure for Barrett’s Esophagus (PROBE): Concept Elicitation and Item Generation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

