Monday Poster Session
Category: Esophagus
P2758 - Re-Examining the Association of Glucagon-Like Peptide-1 Receptor Agonists With Gastroesophageal Reflux Disease Through pH Impedance Testing
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Karissa DiPierro, BS (she/her/hers)
Case Western Reserve University School of Medicine
Cleveland, OH
Presenting Author(s)
Benjamin D. Liu, MD1, Karissa DiPierro, BS2, Kanisha Bahierathan, BS2, Ye Tian, PhD3, Yasir Tarabichi, MD4, Hannah Hill, MSc5, Ronnie Fass, MD, MACG6, Gengqing Song, MD7
1Department of Internal Medicine, Metrohealth Medical Center, Cleveland, OH; 2Case Western Reserve University School of Medicine, Cleveland, OH; 3Cleveland Institute for Computational Biology, Case Western Reserve University, Cleveland, OH; 4Center for Clinical Informatics Research and Education, MetroHealth Medical Center, Cleveland, OH; 5Population Health and Equity Research Institute, Metrohealth Medical Center, Cleveland, OH; 6Division of Gastroenterology and Hepatology, Metrohealth Medical Center, Orange, OH; 7Division of Gastroenterology and Hepatology, Metrohealth Medical Center, Cleveland, OH
Introduction: We previously demonstrated that shorter-acting glucagon-like peptide-1 receptor agonists (GLP-1 RA) are associated with increased risk of new gastroesophageal reflux disease (GERD) or its complications compared to other second-line type 2 diabetes medications (OSLT2DM) in a global population-level database. The aim of this study is to confirm this association in our local database and identify the etiology of this effect through pH impedance testing.
Methods: We identified patients in the Metrohealth database (2006-2025) with T2DM or BMI of ≥ 27 who started a GLP-1 RA (Cohort 1) or OSLT2DM (Cohort 2) using the TriNetX network. Exclusion criteria included history of esophageal motility disorders and major upper abdominal surgery. We extracted patient characteristics, GLP-1 RA dosing and compliance, and pH impedance results. We calculated GERD-related events per 1,000 patient days following index event and short-term GERD-related events while actively on a GLP-1 RA. Events were analyzed with Cox-proportional hazards models. pH impedance results were compared with a t-test and Bonferroni correction for multiple testing.
Results: Cohort 1 had 14,280 patients and Cohort 2 had 19,085. Cohort 1 had 2,506 GERD without esophagitis events at an event rate of 0.3676 per 1,000 patient follow-up days compared to 4,661 GERD without esophagitis events, at 0.0732 events per 1,000 patient follow-up days, in Cohort 2 (HR 5.02; 95% CI 4.78-5.27). Average days until GERD without esophagitis was 521 after index event on GLP-1 RA or 265 while actively on a GLP-1 RA compared to 907 days in Cohort 2. Cohort 1 was more likely to experience GERD and esophagitis, both with bleeding (HR 13.1; 95% CI 4.1-41.2) and without bleeding (HR 4.47; 95% CI 3.92-5.10), GERD and stricture (HR 3.69; 95% CI 2.42-5.63), and Barrett’s esophagus without dysplasia (HR 8.22; 95% CI 5.8-11.7). A total of 12 patients in Cohort 1 and 11 patients in Cohort 2 underwent pH impedance; all patients except 1 were on proton pump inhibitors (PPIs) at time of study. There was no difference in total reflux events (54.0 ± 35.5 vs. 57.4 ± 36.7 in Cohort 2; p = 0.826) or pH< 4% (3.68 ± 5.83 vs. 3.52 ± 5.25; p = 0.944).
Discussion: This study reaffirms that patients on GLP-1 RAs are more likely to develop GERD and its complications. However, GLP-1 RAs do not appear to worsen acid exposure in patients already taking PPIs. Future studies should explore wireless pH monitoring for patients not on PPIs to determine the etiology of worse GERD outcomes.
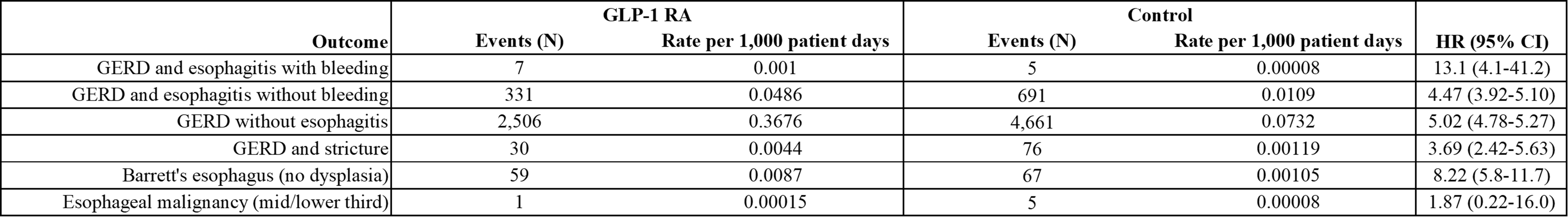
Figure: Figure 1: Risk of GERD Outcomes for Patients on Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) Compared to Other Type 2 Diabetes Medications (Control).
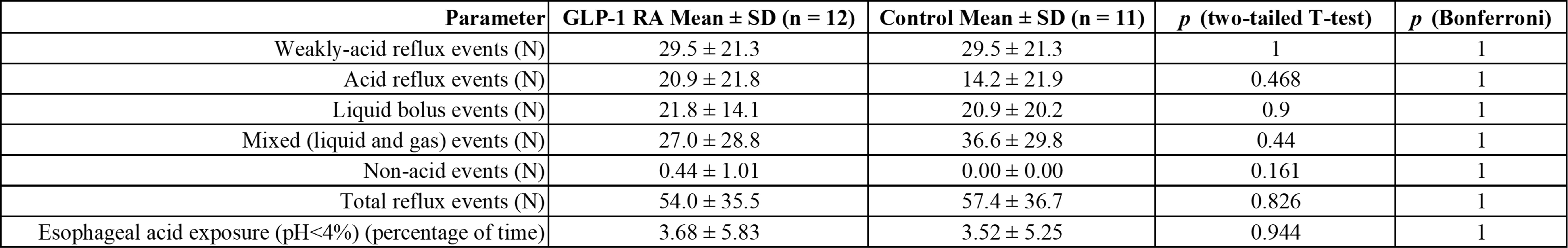
Figure: Figure 2: pH Impedance Results for Patients on Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) Compared to Other Type 2 Diabetes Medications (Control).
Disclosures:
Benjamin Liu indicated no relevant financial relationships.
Karissa DiPierro indicated no relevant financial relationships.
Kanisha Bahierathan indicated no relevant financial relationships.
Ye Tian indicated no relevant financial relationships.
Yasir Tarabichi indicated no relevant financial relationships.
Hannah Hill indicated no relevant financial relationships.
Ronnie Fass: BrainTree Labs/Sebela – Consultant. Carnot – Speaker. Daewoong – Consultant, Speaker. dexcal – Consultant. Megalab – Speaker. Phathom Pharmaceuticals – Advisory Committee/Board Member, Consultant.
Gengqing Song indicated no relevant financial relationships.
Benjamin D. Liu, MD1, Karissa DiPierro, BS2, Kanisha Bahierathan, BS2, Ye Tian, PhD3, Yasir Tarabichi, MD4, Hannah Hill, MSc5, Ronnie Fass, MD, MACG6, Gengqing Song, MD7. P2758 - Re-Examining the Association of Glucagon-Like Peptide-1 Receptor Agonists With Gastroesophageal Reflux Disease Through pH Impedance Testing, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, Metrohealth Medical Center, Cleveland, OH; 2Case Western Reserve University School of Medicine, Cleveland, OH; 3Cleveland Institute for Computational Biology, Case Western Reserve University, Cleveland, OH; 4Center for Clinical Informatics Research and Education, MetroHealth Medical Center, Cleveland, OH; 5Population Health and Equity Research Institute, Metrohealth Medical Center, Cleveland, OH; 6Division of Gastroenterology and Hepatology, Metrohealth Medical Center, Orange, OH; 7Division of Gastroenterology and Hepatology, Metrohealth Medical Center, Cleveland, OH
Introduction: We previously demonstrated that shorter-acting glucagon-like peptide-1 receptor agonists (GLP-1 RA) are associated with increased risk of new gastroesophageal reflux disease (GERD) or its complications compared to other second-line type 2 diabetes medications (OSLT2DM) in a global population-level database. The aim of this study is to confirm this association in our local database and identify the etiology of this effect through pH impedance testing.
Methods: We identified patients in the Metrohealth database (2006-2025) with T2DM or BMI of ≥ 27 who started a GLP-1 RA (Cohort 1) or OSLT2DM (Cohort 2) using the TriNetX network. Exclusion criteria included history of esophageal motility disorders and major upper abdominal surgery. We extracted patient characteristics, GLP-1 RA dosing and compliance, and pH impedance results. We calculated GERD-related events per 1,000 patient days following index event and short-term GERD-related events while actively on a GLP-1 RA. Events were analyzed with Cox-proportional hazards models. pH impedance results were compared with a t-test and Bonferroni correction for multiple testing.
Results: Cohort 1 had 14,280 patients and Cohort 2 had 19,085. Cohort 1 had 2,506 GERD without esophagitis events at an event rate of 0.3676 per 1,000 patient follow-up days compared to 4,661 GERD without esophagitis events, at 0.0732 events per 1,000 patient follow-up days, in Cohort 2 (HR 5.02; 95% CI 4.78-5.27). Average days until GERD without esophagitis was 521 after index event on GLP-1 RA or 265 while actively on a GLP-1 RA compared to 907 days in Cohort 2. Cohort 1 was more likely to experience GERD and esophagitis, both with bleeding (HR 13.1; 95% CI 4.1-41.2) and without bleeding (HR 4.47; 95% CI 3.92-5.10), GERD and stricture (HR 3.69; 95% CI 2.42-5.63), and Barrett’s esophagus without dysplasia (HR 8.22; 95% CI 5.8-11.7). A total of 12 patients in Cohort 1 and 11 patients in Cohort 2 underwent pH impedance; all patients except 1 were on proton pump inhibitors (PPIs) at time of study. There was no difference in total reflux events (54.0 ± 35.5 vs. 57.4 ± 36.7 in Cohort 2; p = 0.826) or pH< 4% (3.68 ± 5.83 vs. 3.52 ± 5.25; p = 0.944).
Discussion: This study reaffirms that patients on GLP-1 RAs are more likely to develop GERD and its complications. However, GLP-1 RAs do not appear to worsen acid exposure in patients already taking PPIs. Future studies should explore wireless pH monitoring for patients not on PPIs to determine the etiology of worse GERD outcomes.

Figure: Figure 1: Risk of GERD Outcomes for Patients on Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) Compared to Other Type 2 Diabetes Medications (Control).

Figure: Figure 2: pH Impedance Results for Patients on Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) Compared to Other Type 2 Diabetes Medications (Control).
Disclosures:
Benjamin Liu indicated no relevant financial relationships.
Karissa DiPierro indicated no relevant financial relationships.
Kanisha Bahierathan indicated no relevant financial relationships.
Ye Tian indicated no relevant financial relationships.
Yasir Tarabichi indicated no relevant financial relationships.
Hannah Hill indicated no relevant financial relationships.
Ronnie Fass: BrainTree Labs/Sebela – Consultant. Carnot – Speaker. Daewoong – Consultant, Speaker. dexcal – Consultant. Megalab – Speaker. Phathom Pharmaceuticals – Advisory Committee/Board Member, Consultant.
Gengqing Song indicated no relevant financial relationships.
Benjamin D. Liu, MD1, Karissa DiPierro, BS2, Kanisha Bahierathan, BS2, Ye Tian, PhD3, Yasir Tarabichi, MD4, Hannah Hill, MSc5, Ronnie Fass, MD, MACG6, Gengqing Song, MD7. P2758 - Re-Examining the Association of Glucagon-Like Peptide-1 Receptor Agonists With Gastroesophageal Reflux Disease Through pH Impedance Testing, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

