Monday Poster Session
Category: Colon
P2447 - Impact of Probiotics on Immune-Related Colitis in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ebubekir Daglilar, MD
Charleston Area Medical Center
Charleston, WV
Presenting Author(s)
Ebubekir Daglilar, MD, Nisar Amin, MD, Tiana Dodd, DO, Cheryl Cox, MD, Harleen Chela, MD
Charleston Area Medical Center, Charleston, WV
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy but are frequently associated with immune-related adverse events (irAEs), particularly in the gastrointestinal (GI) tract. Emerging evidence highlights a strong link between gut microbiota dysbiosis and GI irAEs. Probiotics, widely available as over-the-counter supplements, may modulate gut microbiota and mitigate these effects. This study utilizes the TriNetX database to further explore the impact of probiotics on irAE outcomes in ICI-treated patients, aiming to inform and enhance clinical management strategies.
Methods: This study utilized the TriNetX database, comprising data from over 270 million patients across 120 global healthcare organizations. It included adults (≥18 years) with malignancy treated with PD-1/PD-L1 inhibitors from 2012 to 2024, excluding those with colon cancer, IBD, or bacterial intestinal infections. Two cohorts were created: one comprising patients receiving probiotics and the other including those not receiving probiotics. Propensity score matching (1:1) was conducted using TriNetX’s built-in functions, accounting for demographics, comorbidities, smoking status, BMI, alcohol use, proton pump inhibitor (PPI) use, cancer type, and metastatic disease to ensure balanced cohorts. The primary outcome was the incidence of colitis within 6 months of ICI therapy initiation. Secondary outcomes included hypothyroidism, thyroiditis, hypophysitis, pancreatitis, and skin toxicities. Statistical significance was defined as a 95% confidence interval (CI) and a P-value < 0.05.
Results: A total of 123,410 patients receiving cancer therapy with immune checkpoint inhibitors (ICIs) were identified, of whom 1.4% had a recorded probiotic intake. Two matched cohorts of 1,754 patients each were created. Patients on probiotics demonstrated a significantly lower incidence of colitis (3.2% vs. 4.7%, P = 0.024), hypothyroidism (15.6% vs 18.5%, p=0.025), thyroiditis (4.3% vs 5.9%, p=0.032) compared to those not on probiotics. No significant differences were observed in the incidence of hypophysitis (0.8% vs. 1.0%, p= 0.58), pancreatitis (0.8% vs. 0.8%, P = 1), or skin toxicities (7.5% vs. 7.1%, P = 0.6). (Figure1)
Discussion: Probiotics, a widely used over the counter medication, was found to significantly improve GI and thyroid related adverse events in cancer patients undergoing ICI therapy, with no observed differences in the incidence of other ICI-related immune-related adverse events (irAEs).
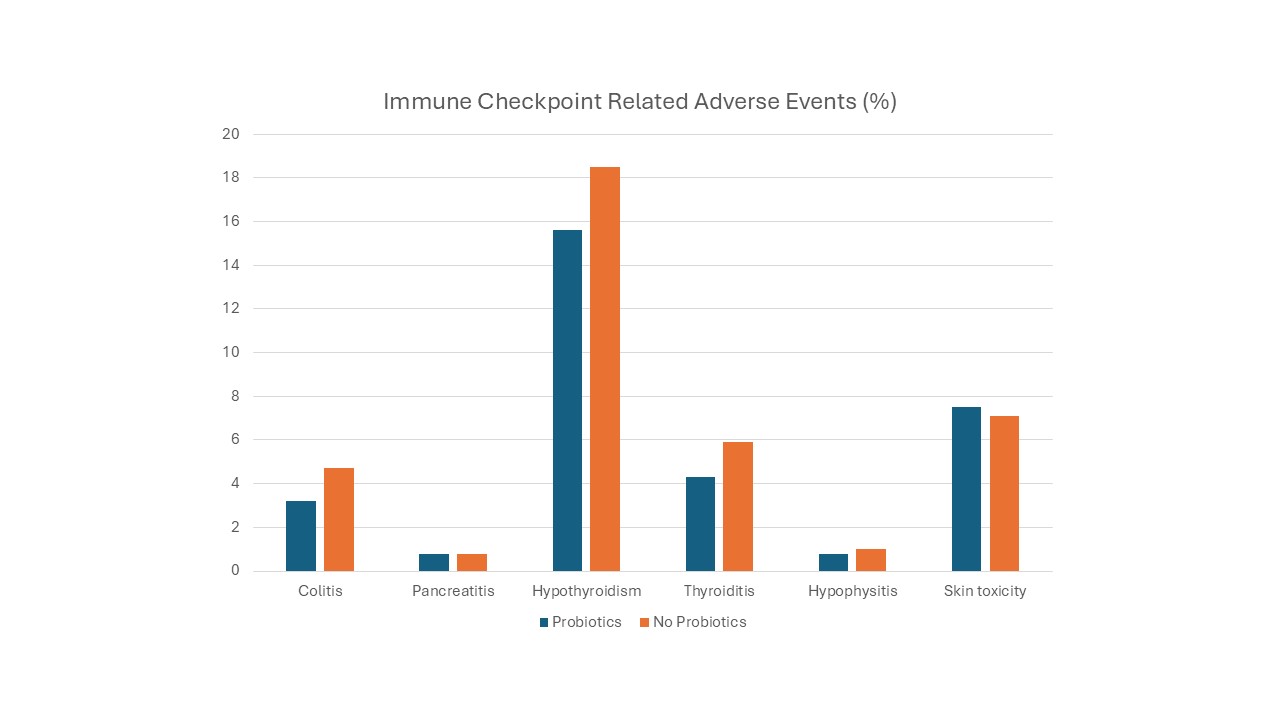
Figure: Figure-1.Immune Checkpoint related adverse event.
Disclosures:
Ebubekir Daglilar indicated no relevant financial relationships.
Nisar Amin indicated no relevant financial relationships.
Tiana Dodd indicated no relevant financial relationships.
Cheryl Cox indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Ebubekir Daglilar, MD, Nisar Amin, MD, Tiana Dodd, DO, Cheryl Cox, MD, Harleen Chela, MD. P2447 - Impact of Probiotics on Immune-Related Colitis in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Charleston Area Medical Center, Charleston, WV
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy but are frequently associated with immune-related adverse events (irAEs), particularly in the gastrointestinal (GI) tract. Emerging evidence highlights a strong link between gut microbiota dysbiosis and GI irAEs. Probiotics, widely available as over-the-counter supplements, may modulate gut microbiota and mitigate these effects. This study utilizes the TriNetX database to further explore the impact of probiotics on irAE outcomes in ICI-treated patients, aiming to inform and enhance clinical management strategies.
Methods: This study utilized the TriNetX database, comprising data from over 270 million patients across 120 global healthcare organizations. It included adults (≥18 years) with malignancy treated with PD-1/PD-L1 inhibitors from 2012 to 2024, excluding those with colon cancer, IBD, or bacterial intestinal infections. Two cohorts were created: one comprising patients receiving probiotics and the other including those not receiving probiotics. Propensity score matching (1:1) was conducted using TriNetX’s built-in functions, accounting for demographics, comorbidities, smoking status, BMI, alcohol use, proton pump inhibitor (PPI) use, cancer type, and metastatic disease to ensure balanced cohorts. The primary outcome was the incidence of colitis within 6 months of ICI therapy initiation. Secondary outcomes included hypothyroidism, thyroiditis, hypophysitis, pancreatitis, and skin toxicities. Statistical significance was defined as a 95% confidence interval (CI) and a P-value < 0.05.
Results: A total of 123,410 patients receiving cancer therapy with immune checkpoint inhibitors (ICIs) were identified, of whom 1.4% had a recorded probiotic intake. Two matched cohorts of 1,754 patients each were created. Patients on probiotics demonstrated a significantly lower incidence of colitis (3.2% vs. 4.7%, P = 0.024), hypothyroidism (15.6% vs 18.5%, p=0.025), thyroiditis (4.3% vs 5.9%, p=0.032) compared to those not on probiotics. No significant differences were observed in the incidence of hypophysitis (0.8% vs. 1.0%, p= 0.58), pancreatitis (0.8% vs. 0.8%, P = 1), or skin toxicities (7.5% vs. 7.1%, P = 0.6). (Figure1)
Discussion: Probiotics, a widely used over the counter medication, was found to significantly improve GI and thyroid related adverse events in cancer patients undergoing ICI therapy, with no observed differences in the incidence of other ICI-related immune-related adverse events (irAEs).

Figure: Figure-1.Immune Checkpoint related adverse event.
Disclosures:
Ebubekir Daglilar indicated no relevant financial relationships.
Nisar Amin indicated no relevant financial relationships.
Tiana Dodd indicated no relevant financial relationships.
Cheryl Cox indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Ebubekir Daglilar, MD, Nisar Amin, MD, Tiana Dodd, DO, Cheryl Cox, MD, Harleen Chela, MD. P2447 - Impact of Probiotics on Immune-Related Colitis in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
