Monday Poster Session
Category: Colon
P2438 - Impact of GLP1RA on Immune-Related Colitis in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Nisar Amin, MD
Charleston Area Medical Center
Charleston, WV
Presenting Author(s)
Nisar Amin, MD1, Mark Ayoub, MD1, Harleen Chela, MD1, Veysel Tahan, MD, FACG2, Ebubekir Daglilar, MD1
1Charleston Area Medical Center, Charleston, WV; 2Northeast Ohio Medical University & CAMC, Charleston, WV
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment but are associated with immune-related adverse events (irAEs) due to excessive immune activation. While irAEs commonly affect the skin, endocrine glands, and gastrointestinal tract, any organ system may be involved. Immune-modulating drugs can influence the incidence and severity of irAEs. Preclinical studies show that GLP-1 receptor agonists (GLP1RAs) have anti-inflammatory properties, as GLP-1 receptor expression is induced by T-cell activation in CD4+ T cells. GLP1RAs have also been shown to reduce surgical needs in IBD, underscoring their role in GI immunity. Although widely used in diabetes, the impact of GLP1RAs on ICI-related irAEs remains unknown. This study investigates their effect in diabetic cancer patients receiving ICIs.
Methods: This retrospective study used the TriNetX database to identify adults (≥18 years) with diabetes and malignancy treated with PD-1/PD-L1 inhibitors between 2012–2024, excluding those with colon cancer, IBD, or intestinal infections. Two cohorts were analyzed: GLP1RA users and those on non-GLP1RA antihyperglycemics. Propensity score matching (1:1) was performed using TriNetX tools, balancing demographics, comorbidities, smoking, BMI, alcohol use, PPI use, cancer type, metastatic status, and glycemic medications. The primary outcome was colitis incidence within 6 months of ICI initiation; secondary outcomes included hypothyroidism, thyroiditis, hypophysitis, pancreatitis, and skin toxicities. Statistical significance was defined as P < 0.05 with 95% confidence intervals.
Results: A total of 15,451 diabetic cancer patients receiving immune checkpoint inhibitors (ICIs) were identified, of whom 7.8% were prescribed GLP1RA. Two matched cohorts of 1,196 patients each were created. Patients on GLP1RA demonstrated a significantly lower incidence of colitis compared to those not on GLP1RA (2.8% vs. 5.4%, P = 0.001). No significant differences were observed in the incidence of hypothyroidism (26% vs. 23%, P = 0.08), thyroiditis (8.1% vs. 6.7%, P = 0.18), hypophysitis (0.9% vs. 0.9%, P = 1), pancreatitis (0.8% vs. 1.2%, P = 0.4), or skin toxicities (11.5% vs. 10.6%, P = 0.47). (Figure1)
Discussion: GLP1RA, a widely used antihyperglycemic medication, was found to significantly improve colitis in cancer patients undergoing ICI therapy, with no observed differences in the incidence of other ICI-related immune-related adverse events (irAEs).
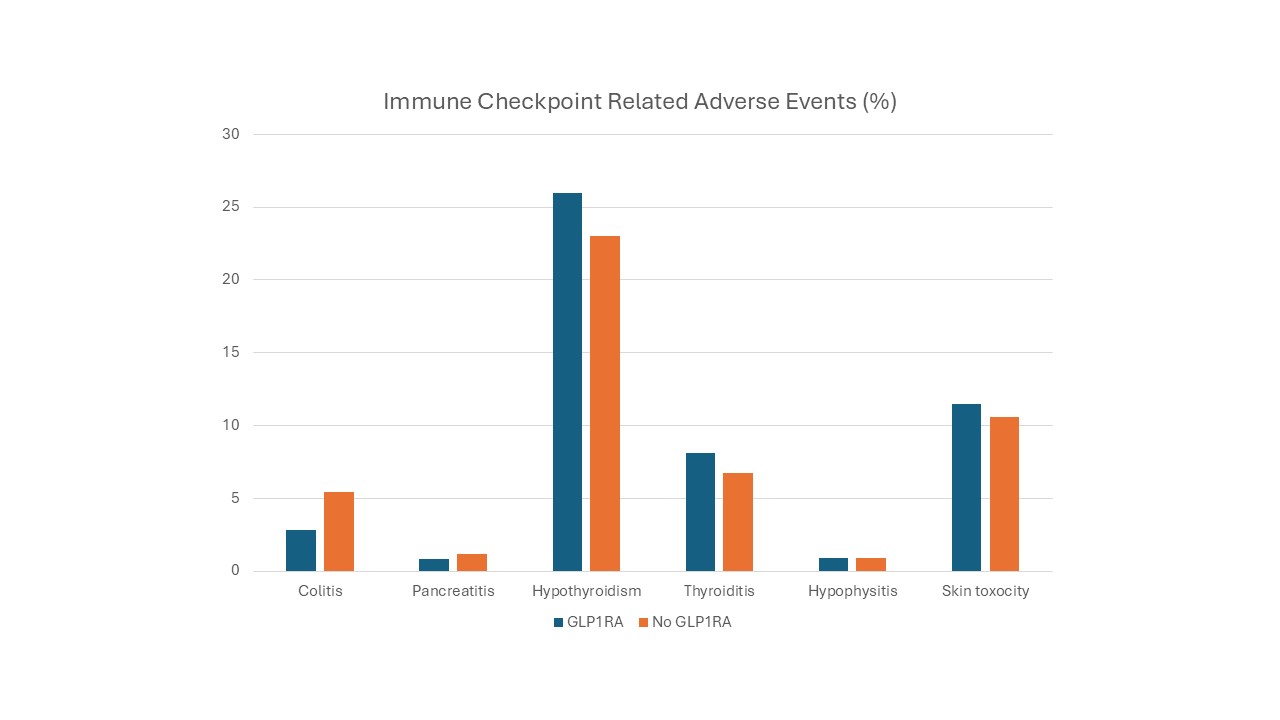
Figure: Figure-1. Immune Checkpoint related adverse events.
Disclosures:
Nisar Amin indicated no relevant financial relationships.
Mark Ayoub indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Veysel Tahan indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Nisar Amin, MD1, Mark Ayoub, MD1, Harleen Chela, MD1, Veysel Tahan, MD, FACG2, Ebubekir Daglilar, MD1. P2438 - Impact of GLP1RA on Immune-Related Colitis in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Charleston Area Medical Center, Charleston, WV; 2Northeast Ohio Medical University & CAMC, Charleston, WV
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment but are associated with immune-related adverse events (irAEs) due to excessive immune activation. While irAEs commonly affect the skin, endocrine glands, and gastrointestinal tract, any organ system may be involved. Immune-modulating drugs can influence the incidence and severity of irAEs. Preclinical studies show that GLP-1 receptor agonists (GLP1RAs) have anti-inflammatory properties, as GLP-1 receptor expression is induced by T-cell activation in CD4+ T cells. GLP1RAs have also been shown to reduce surgical needs in IBD, underscoring their role in GI immunity. Although widely used in diabetes, the impact of GLP1RAs on ICI-related irAEs remains unknown. This study investigates their effect in diabetic cancer patients receiving ICIs.
Methods: This retrospective study used the TriNetX database to identify adults (≥18 years) with diabetes and malignancy treated with PD-1/PD-L1 inhibitors between 2012–2024, excluding those with colon cancer, IBD, or intestinal infections. Two cohorts were analyzed: GLP1RA users and those on non-GLP1RA antihyperglycemics. Propensity score matching (1:1) was performed using TriNetX tools, balancing demographics, comorbidities, smoking, BMI, alcohol use, PPI use, cancer type, metastatic status, and glycemic medications. The primary outcome was colitis incidence within 6 months of ICI initiation; secondary outcomes included hypothyroidism, thyroiditis, hypophysitis, pancreatitis, and skin toxicities. Statistical significance was defined as P < 0.05 with 95% confidence intervals.
Results: A total of 15,451 diabetic cancer patients receiving immune checkpoint inhibitors (ICIs) were identified, of whom 7.8% were prescribed GLP1RA. Two matched cohorts of 1,196 patients each were created. Patients on GLP1RA demonstrated a significantly lower incidence of colitis compared to those not on GLP1RA (2.8% vs. 5.4%, P = 0.001). No significant differences were observed in the incidence of hypothyroidism (26% vs. 23%, P = 0.08), thyroiditis (8.1% vs. 6.7%, P = 0.18), hypophysitis (0.9% vs. 0.9%, P = 1), pancreatitis (0.8% vs. 1.2%, P = 0.4), or skin toxicities (11.5% vs. 10.6%, P = 0.47). (Figure1)
Discussion: GLP1RA, a widely used antihyperglycemic medication, was found to significantly improve colitis in cancer patients undergoing ICI therapy, with no observed differences in the incidence of other ICI-related immune-related adverse events (irAEs).

Figure: Figure-1. Immune Checkpoint related adverse events.
Disclosures:
Nisar Amin indicated no relevant financial relationships.
Mark Ayoub indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Veysel Tahan indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Nisar Amin, MD1, Mark Ayoub, MD1, Harleen Chela, MD1, Veysel Tahan, MD, FACG2, Ebubekir Daglilar, MD1. P2438 - Impact of GLP1RA on Immune-Related Colitis in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
