Monday Poster Session
Category: Biliary/Pancreas
P2226 - Increased Risk of Gallbladder and Biliary Diseases With GLP-1 Receptor Agonists: A US Collaborative Network Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mohammad Kloub, MD (he/him/his)
New York Medical College - Saint Michael's Medical Center
Bloomfield, NJ
Presenting Author(s)
Mohammad Kloub, MD1, Mohamed Eldesouki, MD2, Hazem Abosheaishaa, MD3, Khaled Elfert, MBChB, MRCP4, Omar Abdelhalim, MD5, Ahmed Ibrahim, MD6, Ahmed Salem, MD7, Sherif Andrawes, MD8
1New York Medical College - Saint Michael's Medical Center, Bloomfield, NJ; 2New York Medical College - Saint Michael's Medical Center, Newark, NJ; 3Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY; 4West Virginia University School of Medicine, Morgantown, WV; 5Icahn School of Medicine at Mount Sinai, Queens, NY; 6Medical University of South Carolina, Charleston, SC; 7Maimonides Medical Center, Brooklyn, NY; 8Staten Island University Hospital, Northwell Health, Staten Island, NY
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are increasingly utilized for patients with type 2 diabetes mellitus (T2DM) and obesity. Recent evidence indicates that using GLP-1 RAs could increase the risk of gallbladder (GB) and biliary-related complications such as cholelithiasis and cholecystitis. The purpose of this study is to examine the GB and biliary diseases among T2DM patients treated with GLP-1 RAs compared to those not receiving GLP-1 RAs, using a large real-world dataset.
Methods: A retrospective cohort study was conducted using the TriNet X database. Adults (≥18 years) with T2DM were identified between 2007 and 2020. Patients with history of bariatric surgery, gallbladder and biliary disease, hemolytic anemia, HIV or HIV medications, and pregnancy were excluded. Patients were then stratified into two cohorts: Cohort 1: patient received GLP-1 RAs and Cohort 2: patients received other oral diabetes medications. Propensity score matching (PSM) was utilized to balance baseline characteristics across the cohorts as shown in Table 1. Outcomes were assessed at 2 and 3 years after initiating GLP-1 RAs. Study outcomes were cholelithiasis, choledocholithiasis, cholecystitis, cholangitis, cholecystectomy, or ERCP procedures. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were calculated for each outcome.
Results: Before PSM, there was a total of 32,299 patients in GLP-1 RAs group, while 120,948 patients in control group. After matching, each cohort consisted of 31,524 patients. As shown in Table 2, at the two-year follow-up, patients in the GLP-1 RAs group had a significantly increased risk of cholelithiasis (aOR 1.29; 95% CI: 1.08–1.55; p = 0.005), while the rates of choledocholithiasis, cholecystitis, cholangitis, cholecystectomy, and ERCP procedures were not significant between the cohorts. By three years, the GLP-1 RAs group had significantly greater risks of cholelithiasis (aOR 1.35; 95% CI: 1.32–1.56; p < 0.01), choledocholithiasis (aOR 1.82; 95% CI: 1.10–3.11; p = 0.02), and cholecystitis (aOR 1.42; 95% CI: 1.11–1.87; p = 0.01), whereas the risks of cholangitis and procedural interventions remained non-significant between the cohorts.
Discussion: GLP-1 RAs are associated with increased risk of GB and biliary diseases in patients with T2DM, with this risk increasing over time. These findings underscore the importance of screening patients with high risk for pre-existing gall bladder disease prior to and during GLP-1 RAs therapy.
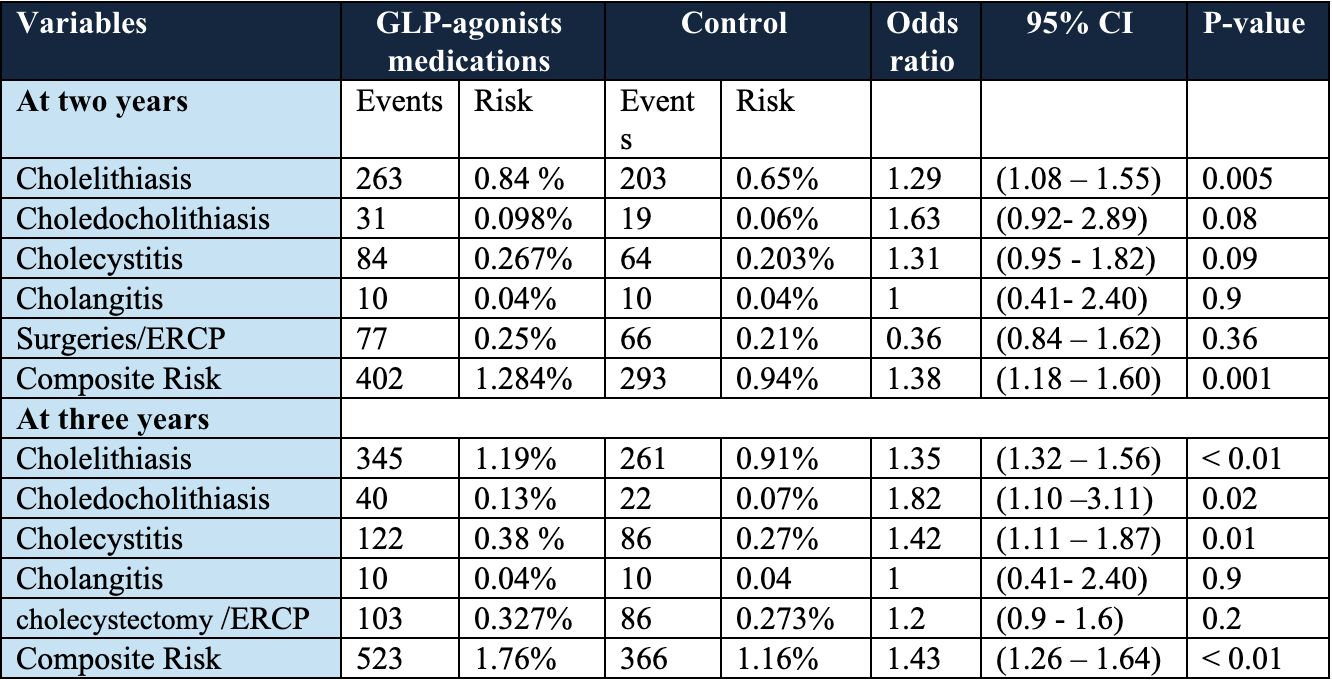
Figure: Table 1 shows Baseline characteristics of the Diabetic patients who are taking GLP-1 RAs plus any oral medications and patients who are taking two or more oral antidiabetic medications
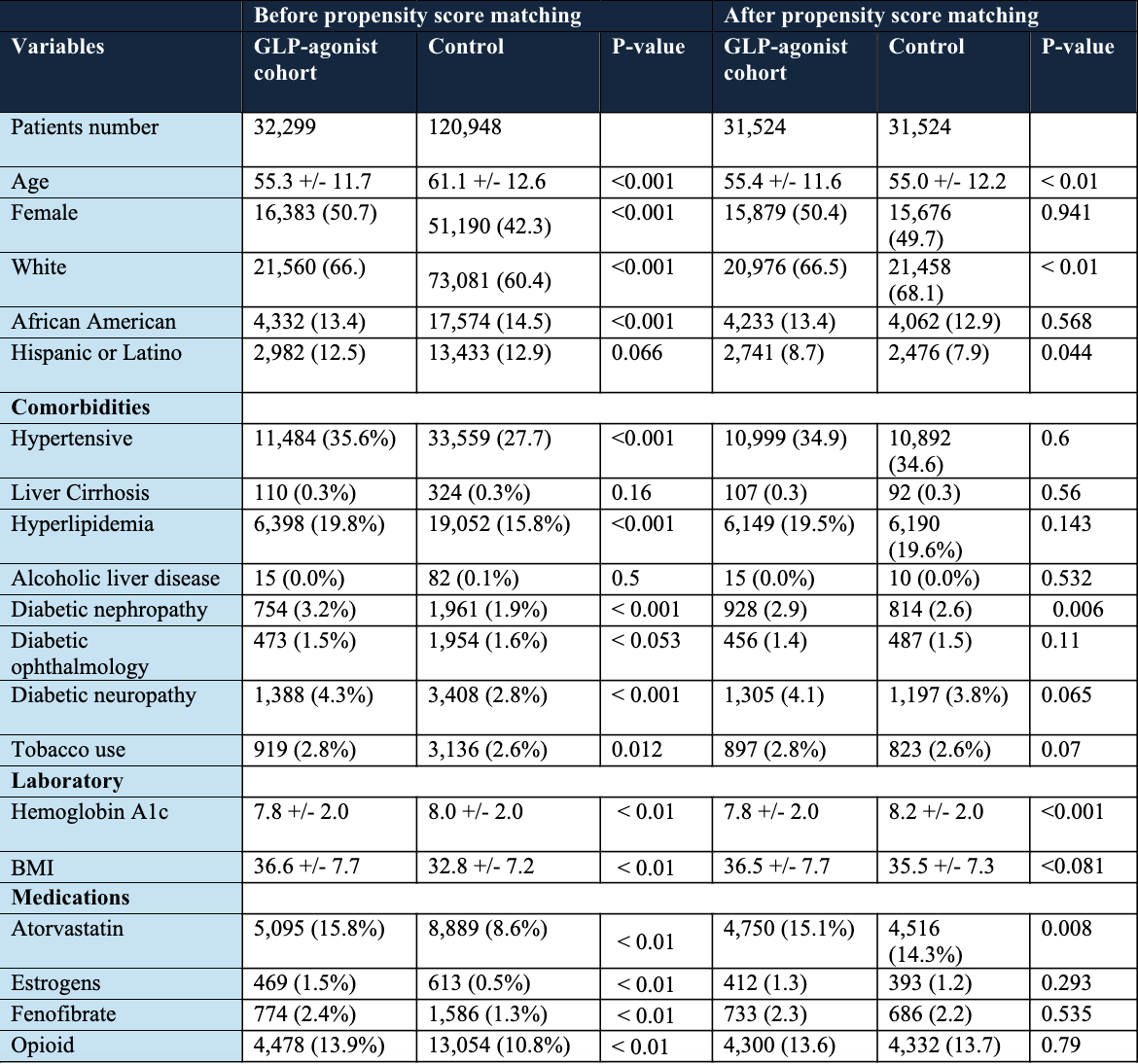
Figure: Table 2 Comparing the outcomes between the two study groups, at two, and three years within starting the GLP-1 RAs
Disclosures:
Mohammad Kloub indicated no relevant financial relationships.
Mohamed Eldesouki indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Omar Abdelhalim indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Sherif Andrawes indicated no relevant financial relationships.
Mohammad Kloub, MD1, Mohamed Eldesouki, MD2, Hazem Abosheaishaa, MD3, Khaled Elfert, MBChB, MRCP4, Omar Abdelhalim, MD5, Ahmed Ibrahim, MD6, Ahmed Salem, MD7, Sherif Andrawes, MD8. P2226 - Increased Risk of Gallbladder and Biliary Diseases With GLP-1 Receptor Agonists: A US Collaborative Network Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1New York Medical College - Saint Michael's Medical Center, Bloomfield, NJ; 2New York Medical College - Saint Michael's Medical Center, Newark, NJ; 3Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY; 4West Virginia University School of Medicine, Morgantown, WV; 5Icahn School of Medicine at Mount Sinai, Queens, NY; 6Medical University of South Carolina, Charleston, SC; 7Maimonides Medical Center, Brooklyn, NY; 8Staten Island University Hospital, Northwell Health, Staten Island, NY
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are increasingly utilized for patients with type 2 diabetes mellitus (T2DM) and obesity. Recent evidence indicates that using GLP-1 RAs could increase the risk of gallbladder (GB) and biliary-related complications such as cholelithiasis and cholecystitis. The purpose of this study is to examine the GB and biliary diseases among T2DM patients treated with GLP-1 RAs compared to those not receiving GLP-1 RAs, using a large real-world dataset.
Methods: A retrospective cohort study was conducted using the TriNet X database. Adults (≥18 years) with T2DM were identified between 2007 and 2020. Patients with history of bariatric surgery, gallbladder and biliary disease, hemolytic anemia, HIV or HIV medications, and pregnancy were excluded. Patients were then stratified into two cohorts: Cohort 1: patient received GLP-1 RAs and Cohort 2: patients received other oral diabetes medications. Propensity score matching (PSM) was utilized to balance baseline characteristics across the cohorts as shown in Table 1. Outcomes were assessed at 2 and 3 years after initiating GLP-1 RAs. Study outcomes were cholelithiasis, choledocholithiasis, cholecystitis, cholangitis, cholecystectomy, or ERCP procedures. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were calculated for each outcome.
Results: Before PSM, there was a total of 32,299 patients in GLP-1 RAs group, while 120,948 patients in control group. After matching, each cohort consisted of 31,524 patients. As shown in Table 2, at the two-year follow-up, patients in the GLP-1 RAs group had a significantly increased risk of cholelithiasis (aOR 1.29; 95% CI: 1.08–1.55; p = 0.005), while the rates of choledocholithiasis, cholecystitis, cholangitis, cholecystectomy, and ERCP procedures were not significant between the cohorts. By three years, the GLP-1 RAs group had significantly greater risks of cholelithiasis (aOR 1.35; 95% CI: 1.32–1.56; p < 0.01), choledocholithiasis (aOR 1.82; 95% CI: 1.10–3.11; p = 0.02), and cholecystitis (aOR 1.42; 95% CI: 1.11–1.87; p = 0.01), whereas the risks of cholangitis and procedural interventions remained non-significant between the cohorts.
Discussion: GLP-1 RAs are associated with increased risk of GB and biliary diseases in patients with T2DM, with this risk increasing over time. These findings underscore the importance of screening patients with high risk for pre-existing gall bladder disease prior to and during GLP-1 RAs therapy.

Figure: Table 1 shows Baseline characteristics of the Diabetic patients who are taking GLP-1 RAs plus any oral medications and patients who are taking two or more oral antidiabetic medications

Figure: Table 2 Comparing the outcomes between the two study groups, at two, and three years within starting the GLP-1 RAs
Disclosures:
Mohammad Kloub indicated no relevant financial relationships.
Mohamed Eldesouki indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Omar Abdelhalim indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Sherif Andrawes indicated no relevant financial relationships.
Mohammad Kloub, MD1, Mohamed Eldesouki, MD2, Hazem Abosheaishaa, MD3, Khaled Elfert, MBChB, MRCP4, Omar Abdelhalim, MD5, Ahmed Ibrahim, MD6, Ahmed Salem, MD7, Sherif Andrawes, MD8. P2226 - Increased Risk of Gallbladder and Biliary Diseases With GLP-1 Receptor Agonists: A US Collaborative Network Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
