Tuesday Poster Session
Category: Colon
P4577 - Adverse Event Profile of Telotristat in Carcinoid Syndrome Diarrhea: Insights From the FAERS Database
- PP
Parth Patel, MD
Ascension Saint Joseph Hospital
Chicago, IL
Presenting Author(s)
1Ascension Saint Joseph Hospital, Chicago, IL; 2Broward Health Medical Center, Fort Lauderdale, FL; 3Saint Vincent Hospital, Worcester, MA
Introduction:
Carcinoid syndrome (CS) diarrhea affects up to 80% of CS patients and poses a significant symptomatic and economic burden. These patients often experience diarrhea and flushing, leading to reduced quality of life and major lifestyle disruptions. Telotristat, a tryptophan hydroxylase inhibitor, was FDA approved in 2017 for CS diarrhea inadequately controlled by somatostatin analogs. Post-marketing data on its safety profile remain limited. In this study, we analyzed adverse drug reactions (ADRs) associated with telotristat using FAERS data to support informed, shared decision-making by prescribers and patients.
Methods:
This study utilized the FDA Adverse Event Reporting System (FAERS) to analyze adverse drug reactions(ADRs) potentially associated with Telotristat use from January 2017 to December 2024. Data were extracted on patient demographics, reported ADRs, and outcomes. Descriptive statistics were used to analyze the ADR reports.
Results:
A total of 5,556 ADR reports were analyzed, including 375 deaths (6.7%) and 838 hospitalizations (15%). Gastrointestinal disorders were most common (47.6%), with diarrhea (14.1%) and constipation (10.5%) frequently reported. Intestinal obstruction occurred in 66 cases (5.7%), 42 (64%) of which were serious. Systemic effects included fatigue (384 cases, 6.9%) and facial or peripheral edema (252 cases, 4.5%). Neurological ADRs accounted for 12.3% of reports, primarily headache and dizziness. Psychiatric symptoms included depression (95 cases, 1.7%) and insomnia (77 cases, 1.4%). Cardiovascular ADRs were reported in 142 cases, with 43% considered serious. There were 452 infection-related ADRs (8.1%), with sepsis classified as serious in 78.6% of cases. Rare but notable events included visual disturbances (21 cases), angioedema/anaphylaxis (5 cases), and acute pancreatitis (4 cases).
Discussion:
Telotristat is generally well tolerated; however, serious adverse events including cardiovascular (e.g., atrial arrhythmias), gastrointestinal (e.g., intestinal obstruction, severe constipation, pancreatitis), and neuropsychiatric effect can occur and add significant morbidity in chronically ill cancer patients. While causality and incidence cannot be established due to database limitations, recognizing potential ADRs is essential. As Telotristat use increases for CS diarrhea, awareness among prescribers and patients is crucial for shared decision making and timely discontinuation if adverse effects emerge.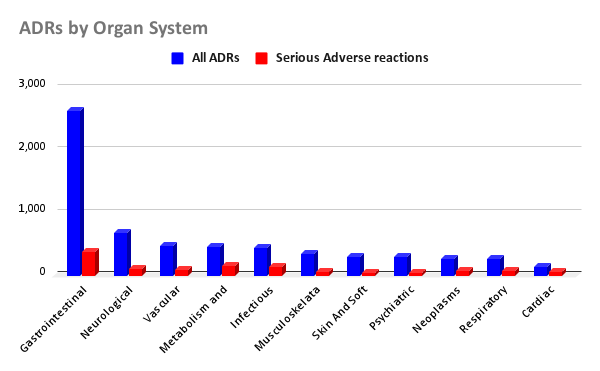
Figure: Figure 1: Reports of adverse drug reactions linked to Telotristat by organ system 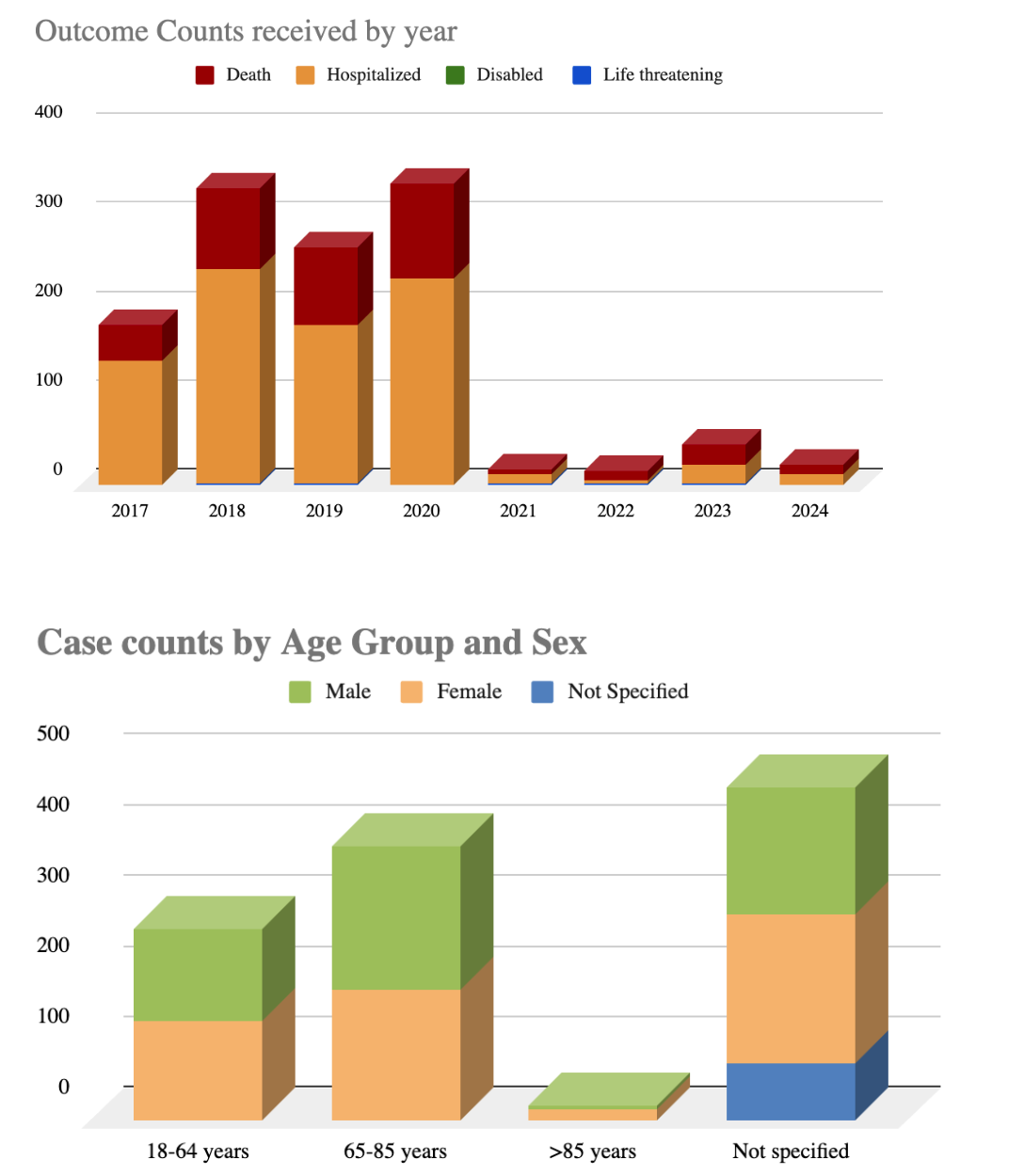
Figure: Figure 2A (above): Reports by year received - Increase in Telotristat ADR reports in 2018 may reflect greater drug usage and awareness after its 2017 approval, along with heightened post-marketing surveillance efforts to assess its safety profile. Figure 2B (below): Reports by Age group and Sex
Disclosures:
Parth Patel indicated no relevant financial relationships.
Manav Patel indicated no relevant financial relationships.
Mythili Menon Pathiyil indicated no relevant financial relationships.
Parth Patel, MD1, Manav Patel, MD2, Mythili Menon Pathiyil, MD3. P4577 - Adverse Event Profile of Telotristat in Carcinoid Syndrome Diarrhea: Insights From the FAERS Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
