Tuesday Poster Session
Category: Colon
P4538 - Severe Colitis in BRAF/MEK Inhibitor Therapy and the Effect of Prior Immune Checkpoint Inhibitor Use: A Case Series and Systematic Review
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Michael Gianarakis, MD
University of Illinois at Chicago
Chicago, IL
Presenting Author(s)
Michael Gianarakis, MD1, Ameen Taufiq, BS2, Rachel Lombard, MD1, Maria El Gemayel, MD3, Itishree Trivedi, MD3
1University of Illinois at Chicago, Chicago, IL; 2Kansas City University, Jacksonville, FL; 3University of Illinois, Chicago, IL
Introduction: BRAF/MEK inhibitors (BRAF/MEKi) are used as first- or second-line therapy for metastatic melanoma and non-small cell lung cancer (NSCLC). While mild gastrointestinal (GI) side effects are common, severe GI toxicity such as colitis is rare and primarily reported in patients with prior immune checkpoint inhibitor (ICI) exposure. We present two cases of severe BRAF/MEKi-associated colitis without prior ICI use and conduct a systematic review to characterize this entity and compare ICI-exposed and non-ICI cases.
Methods: Following PRISMA guidelines, PubMed, EMBASE, and Web of Science were searched from inception to November 2024. Two reviewers independently extracted and summarized data (Table 1).
Case 1: A 62-year-old male with NSCLC on BRAF/MEKi for 3 months developed diarrhea with hematochezia. Colonoscopy revealed two large ileocecal valve (ICV) ulcers with stenosis and erosions, most severe in the right colon and rectum (Figures 1A–B). Biopsy showed eosinophilic infiltration and mucosal ulceration (Figure 1D). Symptoms resolved within a week of drug discontinuation; BRAFi was resumed at half dose without recurrence.
Case 2: A 69-year-old male with NSCLC on BRAF/MEKi for 3 months developed diarrhea and hematochezia. Colonoscopy showed ulcerated ICV and pan-colonic mucosa (Figure 1C). Biopsy revealed active colitis with neutrophil infiltration and focal cryptitis. Symptoms improved after stopping BRAF/MEKi.
Results: Eight studies including 22 patients were identified. Mean age was 65; 91% had melanoma. Colitis occurred significantly later in ICI-exposed patients (6.3 vs. 2.5 months, p=0.025). Right-sided colitis predominated (91%), with 59% isolated to the right colon and 45% involving the ICV. Discontinuation of BRAF/MEKi led to symptom resolution in 94%, while steroids (0/7) and biologics (0/4) were ineffective; two patients required surgery. Among those re-challenged, relapse occurred in 60%; however, no relapse was seen in four patients continued or resumed on BRAFi monotherapy.
Discussion: Severe BRAF/MEKi colitis is a distinct entity marked by ulcerative, right-predominant inflammation, often involving the ICV. MEK inhibition likely disrupts the non-immune-mediated MAPK pathway, essential for GI epithelial survival, leading to mucosal injury. Optimal management involves drug cessation; immunosuppressive therapy is often ineffective. ICI-exposed patients typically present later and fare worse, possibly due to misdiagnosis as ICI colitis and delayed discontinuation of BRAF/MEKi.
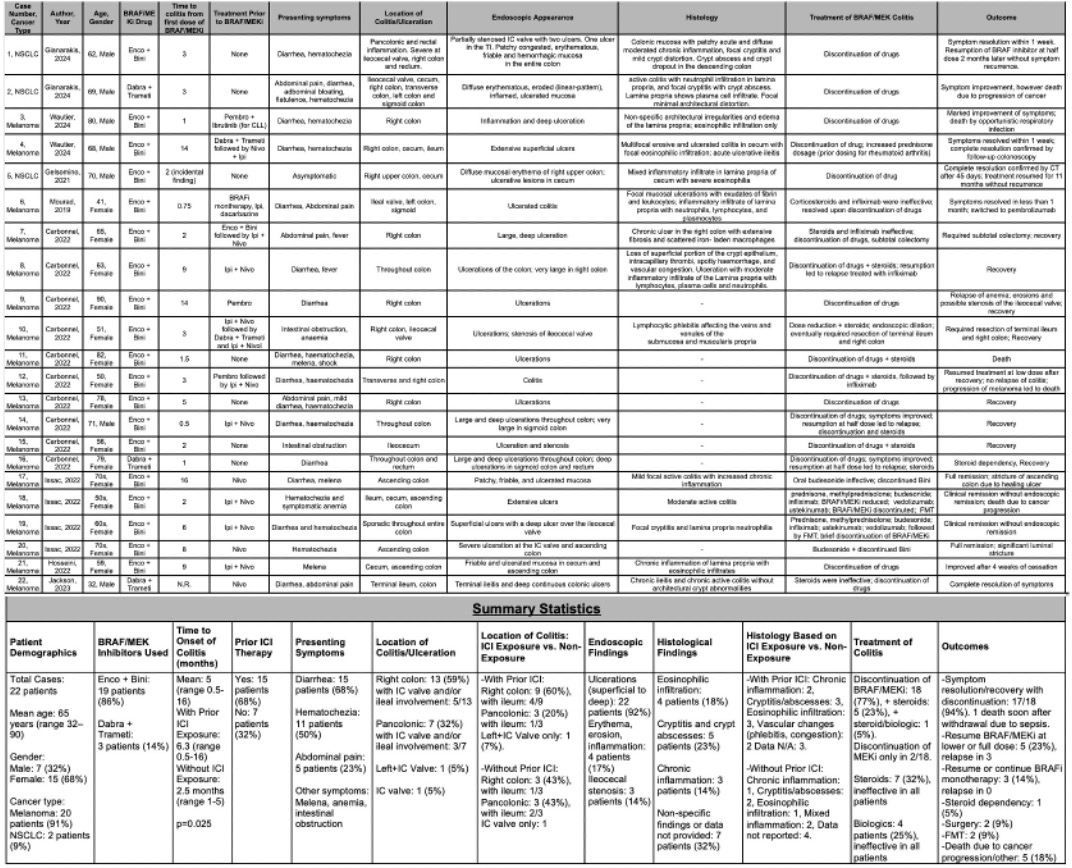
Figure: Figure 1: Endoscopic images showing ileocecal valve ulceration, inflammation and stenosis in patient 1 (A, B). Diffuse area of severely erythematous, eroded and inflamed and ulcerated mucosa in the ascending colon of patient 2 (C). Ileocecal valve biopsy of patient 1 showing lamina propria with eosinophil infiltration and mucosal erosion/ulceration without distortion, cryptitis or crypt abscesses (D).
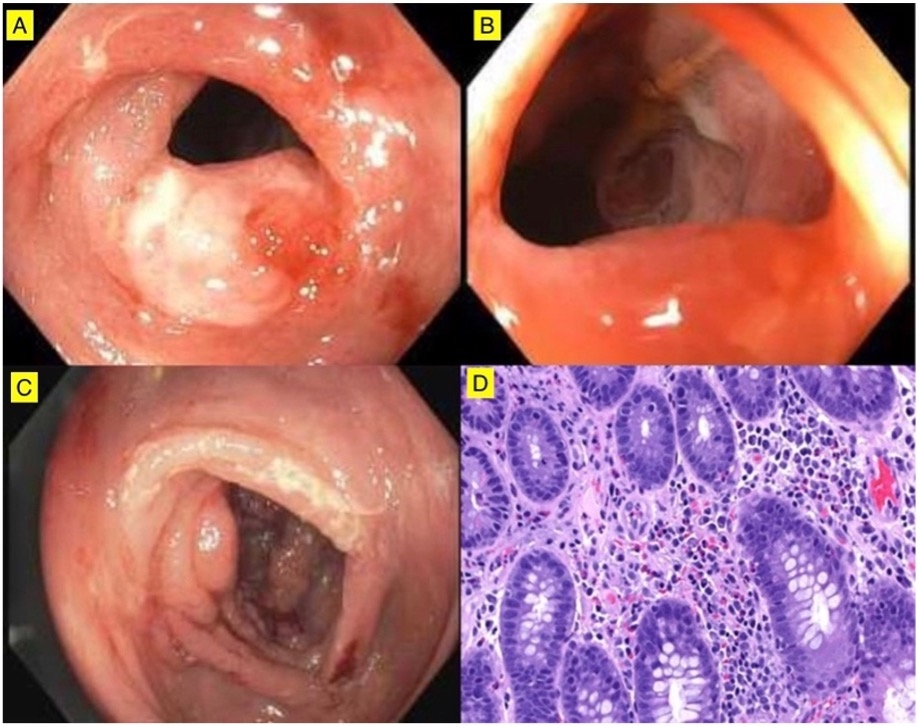
Figure: Table 1: Characteristics, endoscopic/histological findings, treatment and outcomes of patients with severe BRAF/MEKi colitis. (Enco, encorafenib; Bini, binimetinib; Dabra, dabrafenib; Trameti, trametinib; Pembro, pembrolizumab; Nivo, nivolumab; Ipi, ipilimumab; IC, ileocecal; TI, terminal ileum; CLL, chronic lymphocytic leukemia; FMT, fecal microbiota transplant).
Disclosures:
Michael Gianarakis indicated no relevant financial relationships.
Ameen Taufiq indicated no relevant financial relationships.
Rachel Lombard indicated no relevant financial relationships.
Maria El Gemayel indicated no relevant financial relationships.
Itishree Trivedi indicated no relevant financial relationships.
Michael Gianarakis, MD1, Ameen Taufiq, BS2, Rachel Lombard, MD1, Maria El Gemayel, MD3, Itishree Trivedi, MD3. P4538 - Severe Colitis in BRAF/MEK Inhibitor Therapy and the Effect of Prior Immune Checkpoint Inhibitor Use: A Case Series and Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Illinois at Chicago, Chicago, IL; 2Kansas City University, Jacksonville, FL; 3University of Illinois, Chicago, IL
Introduction: BRAF/MEK inhibitors (BRAF/MEKi) are used as first- or second-line therapy for metastatic melanoma and non-small cell lung cancer (NSCLC). While mild gastrointestinal (GI) side effects are common, severe GI toxicity such as colitis is rare and primarily reported in patients with prior immune checkpoint inhibitor (ICI) exposure. We present two cases of severe BRAF/MEKi-associated colitis without prior ICI use and conduct a systematic review to characterize this entity and compare ICI-exposed and non-ICI cases.
Methods: Following PRISMA guidelines, PubMed, EMBASE, and Web of Science were searched from inception to November 2024. Two reviewers independently extracted and summarized data (Table 1).
Case 1: A 62-year-old male with NSCLC on BRAF/MEKi for 3 months developed diarrhea with hematochezia. Colonoscopy revealed two large ileocecal valve (ICV) ulcers with stenosis and erosions, most severe in the right colon and rectum (Figures 1A–B). Biopsy showed eosinophilic infiltration and mucosal ulceration (Figure 1D). Symptoms resolved within a week of drug discontinuation; BRAFi was resumed at half dose without recurrence.
Case 2: A 69-year-old male with NSCLC on BRAF/MEKi for 3 months developed diarrhea and hematochezia. Colonoscopy showed ulcerated ICV and pan-colonic mucosa (Figure 1C). Biopsy revealed active colitis with neutrophil infiltration and focal cryptitis. Symptoms improved after stopping BRAF/MEKi.
Results: Eight studies including 22 patients were identified. Mean age was 65; 91% had melanoma. Colitis occurred significantly later in ICI-exposed patients (6.3 vs. 2.5 months, p=0.025). Right-sided colitis predominated (91%), with 59% isolated to the right colon and 45% involving the ICV. Discontinuation of BRAF/MEKi led to symptom resolution in 94%, while steroids (0/7) and biologics (0/4) were ineffective; two patients required surgery. Among those re-challenged, relapse occurred in 60%; however, no relapse was seen in four patients continued or resumed on BRAFi monotherapy.
Discussion: Severe BRAF/MEKi colitis is a distinct entity marked by ulcerative, right-predominant inflammation, often involving the ICV. MEK inhibition likely disrupts the non-immune-mediated MAPK pathway, essential for GI epithelial survival, leading to mucosal injury. Optimal management involves drug cessation; immunosuppressive therapy is often ineffective. ICI-exposed patients typically present later and fare worse, possibly due to misdiagnosis as ICI colitis and delayed discontinuation of BRAF/MEKi.

Figure: Figure 1: Endoscopic images showing ileocecal valve ulceration, inflammation and stenosis in patient 1 (A, B). Diffuse area of severely erythematous, eroded and inflamed and ulcerated mucosa in the ascending colon of patient 2 (C). Ileocecal valve biopsy of patient 1 showing lamina propria with eosinophil infiltration and mucosal erosion/ulceration without distortion, cryptitis or crypt abscesses (D).

Figure: Table 1: Characteristics, endoscopic/histological findings, treatment and outcomes of patients with severe BRAF/MEKi colitis. (Enco, encorafenib; Bini, binimetinib; Dabra, dabrafenib; Trameti, trametinib; Pembro, pembrolizumab; Nivo, nivolumab; Ipi, ipilimumab; IC, ileocecal; TI, terminal ileum; CLL, chronic lymphocytic leukemia; FMT, fecal microbiota transplant).
Disclosures:
Michael Gianarakis indicated no relevant financial relationships.
Ameen Taufiq indicated no relevant financial relationships.
Rachel Lombard indicated no relevant financial relationships.
Maria El Gemayel indicated no relevant financial relationships.
Itishree Trivedi indicated no relevant financial relationships.
Michael Gianarakis, MD1, Ameen Taufiq, BS2, Rachel Lombard, MD1, Maria El Gemayel, MD3, Itishree Trivedi, MD3. P4538 - Severe Colitis in BRAF/MEK Inhibitor Therapy and the Effect of Prior Immune Checkpoint Inhibitor Use: A Case Series and Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
