Tuesday Poster Session
Category: Biliary/Pancreas
P4376 - Trapezoid RX Wire Guided Retrieval Basket Device Related Complications: Food and Drug Administration Database Analysis Involving Manufacturer and User Facility Device Experience
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Fnu Arti, MD (she/her/hers)
Saint Francis Medical Center
Monroe, LA
Presenting Author(s)
Fnu Arti, MD1, Aaphtaab Dheendsa, MD1, Rahul Robaish. Kumar, MD1, Aasta Kumari, MD2, Fnu Aakash, MD3, Rahul Kumar, MD4, Sunny Kumar, MD5, Osama Abdur Rehman, MD3, Nikil Kumar, 6, Fnu Anjali, 7, Abir Islam, 8
1Saint Francis Medical Center, Monroe, LA; 2North Central Bronx Hospital, New York, NY; 3Florida State University, Cape Coral, FL; 4North Central Bronx Hospital, Bronx, NY; 5Wright Center for Graduate Medical Education, Scranton, PA; 6Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 7Bahria university of health sciences, Karachi, Sindh, Pakistan; 8Edward Via College of Osteopathic Medicine, Monroe, LA
Introduction: According to the American Society for Gastrointestinal Endoscopy (ASGE), approximately 450,000-500,000 Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures are performed annually in the United States for diagnostic and therapeutic biliary and pancreatic interventions. Although pancreatitis and bleeding are the most common procedural complications, device-related issues account for a notable 5-10% of adverse events. Our study analyzed post-marketing surveillance data from the Food and Drug Administration's (FDA) Manufacturer and User Facility Device Experience (MAUDE) database to evaluate complications associated with widely used Trapezoid RX Wire Guided Retrieval Basket (Trapezoid) during ERCP.
Methods: A systematic search of FDA’s MAUDE database for Trapezoid reports from 2014-2024 was conducted. After excluding duplicates and incomplete entries, 500 adverse events were analyzed. Data were categorized by event type, device problem, and patient complications, with stratification by manufacturer-confirmed errors.
Results: We reviewed 500 MAUDE database reports related to the Trapezoid device and classified adverse events into two categories: device failures and patient complications. The most common device-related issues were breakage (52.5%), material deformation (23.2%), and malfunction (15.6%) [Fig 1]. Patient-related complications were less frequent, including foreign body retention (1.6%), surgical retrieval of retained devices (1.2%), hemorrhage (0.8%), and a single case of fatal hepatic pseudoaneurysm (0.2%) [Fig 2].
Discussion: The trapezoid is specifically engineered to fragment and extract biliary stones during the ERCP, combining flexible navigation with rigid retrieval capabilities enhances complex biliary access, cannulation and needle-knife-free precut sphincterotomies. Analysis of MAUDE database reports highlights critical safety concerns, including device fractures, material deformations, and functional failures that demand immediate quality control measures. Additionally, the recurring issue of retained foreign bodies often necessitating repeat interventions or even exploratory surgery highlights the urgent need for enhanced procedural oversight. Moving forward, optimizing the trapezoid device's clinical utility will require dual focus: reinforcing device resilience to prevent mechanical failures and implementing standard safety guidelines to minimize complications, thereby elevating both patient safety and procedural efficacy in ERCP interventions.
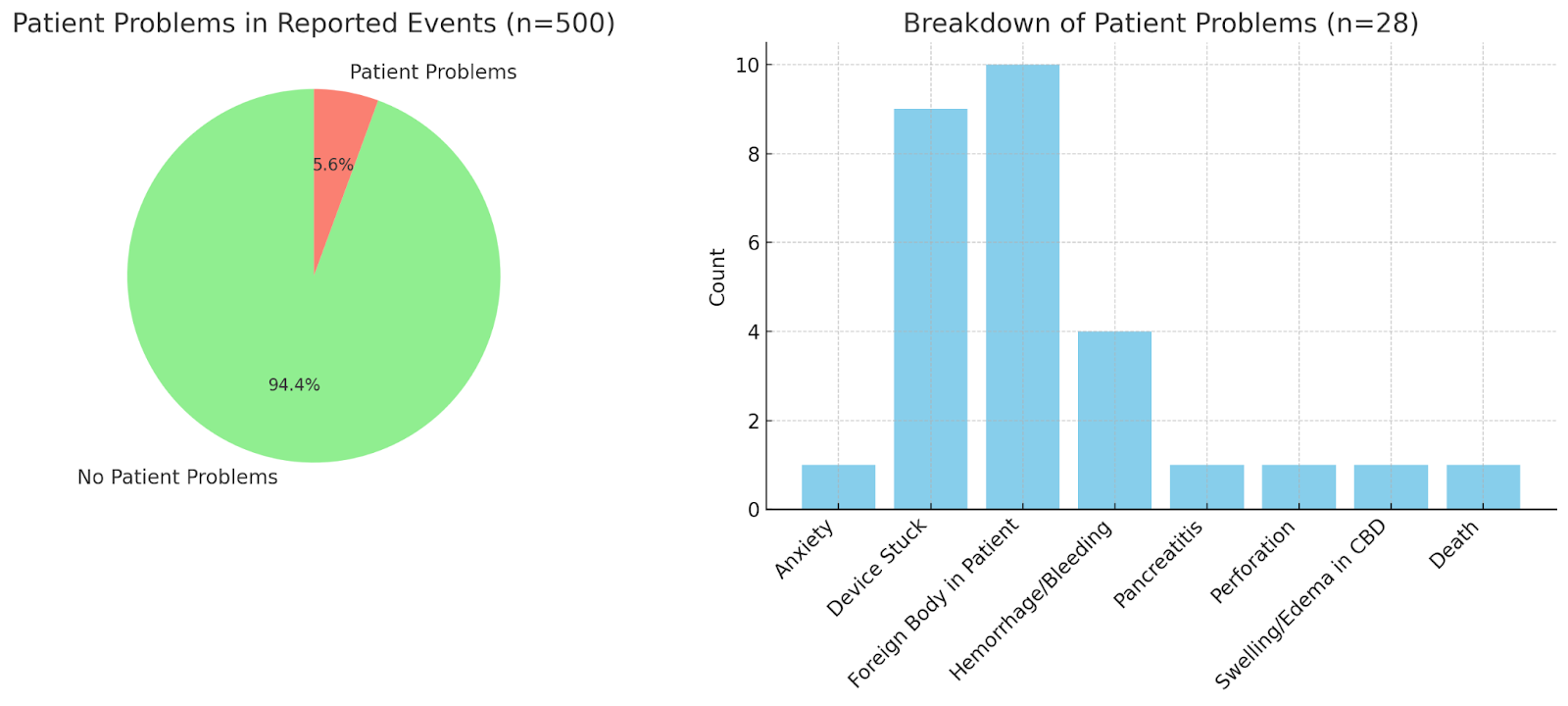
Figure: Figure 1 highlighting device related complications of Trapezoid Device
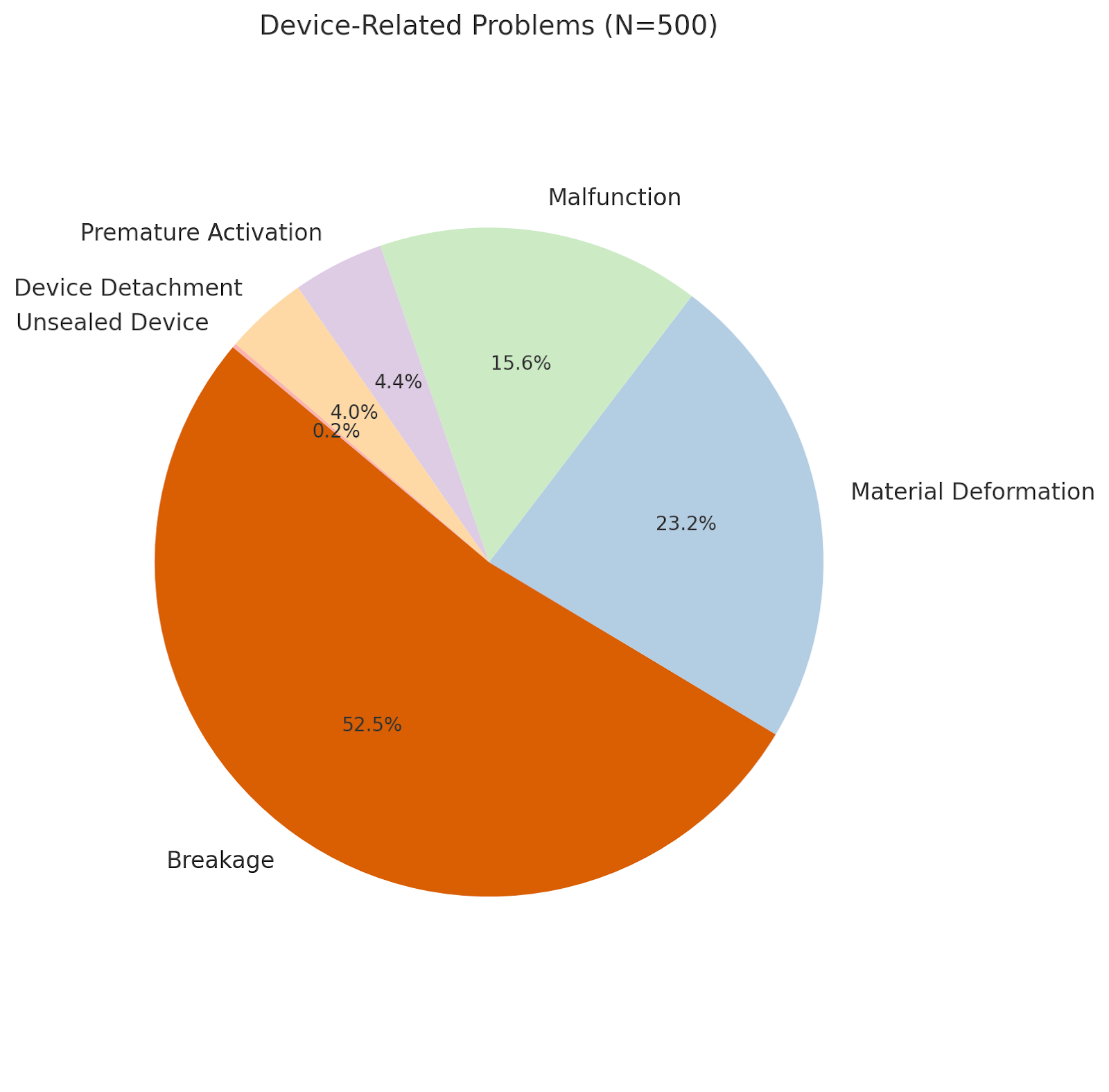
Figure: Figure 2 highlighting patient related complications from Trapezoid device.
Disclosures:
Fnu Arti indicated no relevant financial relationships.
Aaphtaab Dheendsa indicated no relevant financial relationships.
Rahul Kumar indicated no relevant financial relationships.
Aasta Kumari indicated no relevant financial relationships.
Fnu Aakash indicated no relevant financial relationships.
Rahul Kumar indicated no relevant financial relationships.
Sunny Kumar indicated no relevant financial relationships.
Osama Abdur Rehman indicated no relevant financial relationships.
Nikil Kumar indicated no relevant financial relationships.
Fnu Anjali indicated no relevant financial relationships.
Abir Islam indicated no relevant financial relationships.
Fnu Arti, MD1, Aaphtaab Dheendsa, MD1, Rahul Robaish. Kumar, MD1, Aasta Kumari, MD2, Fnu Aakash, MD3, Rahul Kumar, MD4, Sunny Kumar, MD5, Osama Abdur Rehman, MD3, Nikil Kumar, 6, Fnu Anjali, 7, Abir Islam, 8. P4376 - Trapezoid RX Wire Guided Retrieval Basket Device Related Complications: Food and Drug Administration Database Analysis Involving Manufacturer and User Facility Device Experience, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Saint Francis Medical Center, Monroe, LA; 2North Central Bronx Hospital, New York, NY; 3Florida State University, Cape Coral, FL; 4North Central Bronx Hospital, Bronx, NY; 5Wright Center for Graduate Medical Education, Scranton, PA; 6Liaquat University of Medical and Health Science, Hyderabad, Sindh, Pakistan; 7Bahria university of health sciences, Karachi, Sindh, Pakistan; 8Edward Via College of Osteopathic Medicine, Monroe, LA
Introduction: According to the American Society for Gastrointestinal Endoscopy (ASGE), approximately 450,000-500,000 Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures are performed annually in the United States for diagnostic and therapeutic biliary and pancreatic interventions. Although pancreatitis and bleeding are the most common procedural complications, device-related issues account for a notable 5-10% of adverse events. Our study analyzed post-marketing surveillance data from the Food and Drug Administration's (FDA) Manufacturer and User Facility Device Experience (MAUDE) database to evaluate complications associated with widely used Trapezoid RX Wire Guided Retrieval Basket (Trapezoid) during ERCP.
Methods: A systematic search of FDA’s MAUDE database for Trapezoid reports from 2014-2024 was conducted. After excluding duplicates and incomplete entries, 500 adverse events were analyzed. Data were categorized by event type, device problem, and patient complications, with stratification by manufacturer-confirmed errors.
Results: We reviewed 500 MAUDE database reports related to the Trapezoid device and classified adverse events into two categories: device failures and patient complications. The most common device-related issues were breakage (52.5%), material deformation (23.2%), and malfunction (15.6%) [Fig 1]. Patient-related complications were less frequent, including foreign body retention (1.6%), surgical retrieval of retained devices (1.2%), hemorrhage (0.8%), and a single case of fatal hepatic pseudoaneurysm (0.2%) [Fig 2].
Discussion: The trapezoid is specifically engineered to fragment and extract biliary stones during the ERCP, combining flexible navigation with rigid retrieval capabilities enhances complex biliary access, cannulation and needle-knife-free precut sphincterotomies. Analysis of MAUDE database reports highlights critical safety concerns, including device fractures, material deformations, and functional failures that demand immediate quality control measures. Additionally, the recurring issue of retained foreign bodies often necessitating repeat interventions or even exploratory surgery highlights the urgent need for enhanced procedural oversight. Moving forward, optimizing the trapezoid device's clinical utility will require dual focus: reinforcing device resilience to prevent mechanical failures and implementing standard safety guidelines to minimize complications, thereby elevating both patient safety and procedural efficacy in ERCP interventions.

Figure: Figure 1 highlighting device related complications of Trapezoid Device

Figure: Figure 2 highlighting patient related complications from Trapezoid device.
Disclosures:
Fnu Arti indicated no relevant financial relationships.
Aaphtaab Dheendsa indicated no relevant financial relationships.
Rahul Kumar indicated no relevant financial relationships.
Aasta Kumari indicated no relevant financial relationships.
Fnu Aakash indicated no relevant financial relationships.
Rahul Kumar indicated no relevant financial relationships.
Sunny Kumar indicated no relevant financial relationships.
Osama Abdur Rehman indicated no relevant financial relationships.
Nikil Kumar indicated no relevant financial relationships.
Fnu Anjali indicated no relevant financial relationships.
Abir Islam indicated no relevant financial relationships.
Fnu Arti, MD1, Aaphtaab Dheendsa, MD1, Rahul Robaish. Kumar, MD1, Aasta Kumari, MD2, Fnu Aakash, MD3, Rahul Kumar, MD4, Sunny Kumar, MD5, Osama Abdur Rehman, MD3, Nikil Kumar, 6, Fnu Anjali, 7, Abir Islam, 8. P4376 - Trapezoid RX Wire Guided Retrieval Basket Device Related Complications: Food and Drug Administration Database Analysis Involving Manufacturer and User Facility Device Experience, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

