Tuesday Poster Session
Category: Biliary/Pancreas
P4318 - Cholelithiasis as a Possible Drug Reaction in Use of Resmiteron
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Krishna Shah, MD
Advocate Christ Medical Center
Chicago, IL
Presenting Author(s)
Krishna Shah, MD1, Nick Adimi, MD, MS, BS2, Rogelio Silva, MD3
1Advocate Christ Medical Center, Chicago, IL; 2Advocate Lutheran General, Park Ridge, IL; 3Advocate Christ Medical Center, Oak Lawn, IL
Introduction: Resmetirom is a recently approved thyroid hormone receptor-beta agonist used for the treatment of metabolic dysfunction-associated fatty liver disease (MAFLD). As the incidence of MAFLD-related cirrhosis rises, resmetirom offers a promising therapeutic option, showing encouraging results in early clinical trials and emerging real-world use [1]. In this report, we present data suggesting a potential association between resmetirom and cholelithiasis.
Methods: The Food and Drug Adverse Events Reporting System (FAERS) database was used to review the Adverse Drug Reactions (ADRs) for this study. The database is made up of voluntarily reported ADRs used for post marketing data. Of the 30,668,520 reports from January 1968 to March 2025, 405 of them were reports for resmiteron. The OpenVigil FDA program was used to assess these ADRs using reporting odds ratio (ROR). The formula is as follows:
ROR = (a/c)/(b/d)
a:=drug with ADR, b:=all drugs with ADR, c=all ADRs for the drug, d=other drugs with other ADRs. 95% confidence intervals were calculated. A ROR >1 indicates an ADR frequency is not occurring by chance alone and suggests a significant post-marketing signal.
Results: There were 3 reports of cholelithiasis and 1 report of cholecystitis with usage of resmiteron. The ADR of cholelithiasis was seen to be statistically significant with an ROR of 4.75 (CI 1.52-14.79) while the ADR of cholecystitis was seen to not be statistically significant with a ROR of 4.39 (CI 0.62-31.26).
Discussion: The full spectrum of a drug's safety profile often emerges only after widespread use in diverse patient populations. The ADR of cholelithiasis in the use of Resmitron was seen to be statistically significant. While cholecystitis was seen in only one patient on 405 the future implication here will be to see whether this association with cholelithiasis results in increased episodes of biliary colic, cholecystitis and further complications within the biliary tree. The mechanism by which cholelithiasis is associated with resmetirom is not well understood however thyroid hormone is known to alter bile acid metabolism which can create a favorable environment for gallstone formation [2]. While there is possible association, it will be important to consider screening for gallstone formation as a part of surveillance while taking resmetirom.
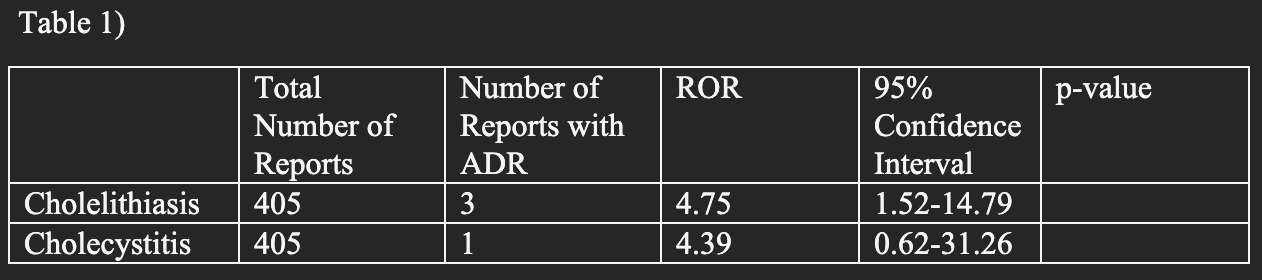
Figure: Table 1: Highlighting total number of ADRs reported, cholelithiasis and cholecystitis ADRs with their corresponding ROR and 95% confidence interval
Disclosures:
Krishna Shah indicated no relevant financial relationships.
Nick Adimi indicated no relevant financial relationships.
Rogelio Silva indicated no relevant financial relationships.
Krishna Shah, MD1, Nick Adimi, MD, MS, BS2, Rogelio Silva, MD3. P4318 - Cholelithiasis as a Possible Drug Reaction in Use of Resmiteron, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Advocate Christ Medical Center, Chicago, IL; 2Advocate Lutheran General, Park Ridge, IL; 3Advocate Christ Medical Center, Oak Lawn, IL
Introduction: Resmetirom is a recently approved thyroid hormone receptor-beta agonist used for the treatment of metabolic dysfunction-associated fatty liver disease (MAFLD). As the incidence of MAFLD-related cirrhosis rises, resmetirom offers a promising therapeutic option, showing encouraging results in early clinical trials and emerging real-world use [1]. In this report, we present data suggesting a potential association between resmetirom and cholelithiasis.
Methods: The Food and Drug Adverse Events Reporting System (FAERS) database was used to review the Adverse Drug Reactions (ADRs) for this study. The database is made up of voluntarily reported ADRs used for post marketing data. Of the 30,668,520 reports from January 1968 to March 2025, 405 of them were reports for resmiteron. The OpenVigil FDA program was used to assess these ADRs using reporting odds ratio (ROR). The formula is as follows:
ROR = (a/c)/(b/d)
a:=drug with ADR, b:=all drugs with ADR, c=all ADRs for the drug, d=other drugs with other ADRs. 95% confidence intervals were calculated. A ROR >1 indicates an ADR frequency is not occurring by chance alone and suggests a significant post-marketing signal.
Results: There were 3 reports of cholelithiasis and 1 report of cholecystitis with usage of resmiteron. The ADR of cholelithiasis was seen to be statistically significant with an ROR of 4.75 (CI 1.52-14.79) while the ADR of cholecystitis was seen to not be statistically significant with a ROR of 4.39 (CI 0.62-31.26).
Discussion: The full spectrum of a drug's safety profile often emerges only after widespread use in diverse patient populations. The ADR of cholelithiasis in the use of Resmitron was seen to be statistically significant. While cholecystitis was seen in only one patient on 405 the future implication here will be to see whether this association with cholelithiasis results in increased episodes of biliary colic, cholecystitis and further complications within the biliary tree. The mechanism by which cholelithiasis is associated with resmetirom is not well understood however thyroid hormone is known to alter bile acid metabolism which can create a favorable environment for gallstone formation [2]. While there is possible association, it will be important to consider screening for gallstone formation as a part of surveillance while taking resmetirom.
1. Harrison SA, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. 2024
2. Soares De Oliveira L, et al. Thyroid hormone and the Liver. 2024

Figure: Table 1: Highlighting total number of ADRs reported, cholelithiasis and cholecystitis ADRs with their corresponding ROR and 95% confidence interval
Disclosures:
Krishna Shah indicated no relevant financial relationships.
Nick Adimi indicated no relevant financial relationships.
Rogelio Silva indicated no relevant financial relationships.
Krishna Shah, MD1, Nick Adimi, MD, MS, BS2, Rogelio Silva, MD3. P4318 - Cholelithiasis as a Possible Drug Reaction in Use of Resmiteron, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
