Sunday Poster Session
Category: Small Intestine
P1956 - A Rare Complication of Poorly Controlled Celiac Disease: Duodenal Obstruction Secondary to Combined B and T Cell Lymphoma
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- KG
Kanika Garg, MD
Rush University Medical Center
Chicago, IL
Presenting Author(s)
Award: ACG Presidential Poster Award
Kanika Garg, MD, Agnieszka Maniak, MD, Thomas J. Wang, MD, Christopher G. Chapman, MD, Ajaypal Singh, MD, Irving Waxman, MD, FACG, Neal A. Mehta, MD
Rush University Medical Center, Chicago, IL
Introduction: Celiac disease carries an increased risk of lymphoproliferative disorders, particularly enteropathy-associated T-cell lymphoma (EATL) with a 50-fold increased risk. Diffuse large B-cell lymphoma (DLBCL) occurs at a 3-fold higher rate. Strict adherence to a gluten-free diet is crucial for reducing malignant complications.
Case Description/
Methods: A 62-year-old Spanish-speaking female presented in 2011 with iron deficiency anemia (hemoglobin 10.3 g/dL), abdominal pain, and vitamin D deficiency. Anti-tissue transglutaminase and endomysial antibodies were positive. Esophagogastroduodenoscopy (EGD) demonstrated atrophic mucosa, blunted villi, and luminal narrowing in the duodenum. Pathology confirmed celiac disease (Marsh IIIC). Despite extensive counseling, she was lost to follow-up.
Fourteen years later, without any interval follow-up, she presented with abdominal distension, nausea, and vomiting. She denied adhering to a gluten-free diet. Labs demonstrated worsening anemia (hemoglobin 6.8 g/dL), hypoalbuminemia (albumin 2.4 g/dL), and mild transaminase elevations (AST 55 U/L, ALT 110 U/L). Computed tomography (CT) revealed a 4.3 x 2.4 cm lobulated soft tissue lesion at the hepatic hilum extending distally with circumferential duodenal thickening, resulting in gastric outlet obstruction. CT also showed extensive lymphadenopathy.
EGD and endoscopic ultrasound (EUS) showed complete duodenal bulb obstruction with wall thickening and malignant-appearing porta hepatis lymph nodes (Figure 1, Figure 2). Duodenal and lymph node biopsies were positive for both T and B cell lymphomas. Immunohistochemistry revealed CD20-positive cells and CD3-positive T cells. After further pathologic evaluation and multidisciplinary discussion, she was diagnosed with DLBCL with a subset of T cell population secondary to T cell activation in the setting of long-standing, poorly controlled celiac disease. Bone marrow biopsy confirmed advanced-stage disease.
With conservative management of the obstruction and initiation of inpatient chemotherapy, her duodenal stricture rapidly resolved, allowing her to resume enteral feeding within one week.
Discussion: This case illustrates a rare malignant obstruction from combined DLBCL with T-cell activation in poorly controlled celiac disease. It underscores the importance of dietary adherence and regular follow-up in celiac patients to prevent malignant complications, as well as the diagnostic utility of EUS-guided tissue acquisition in complex lymphoproliferative disorders.
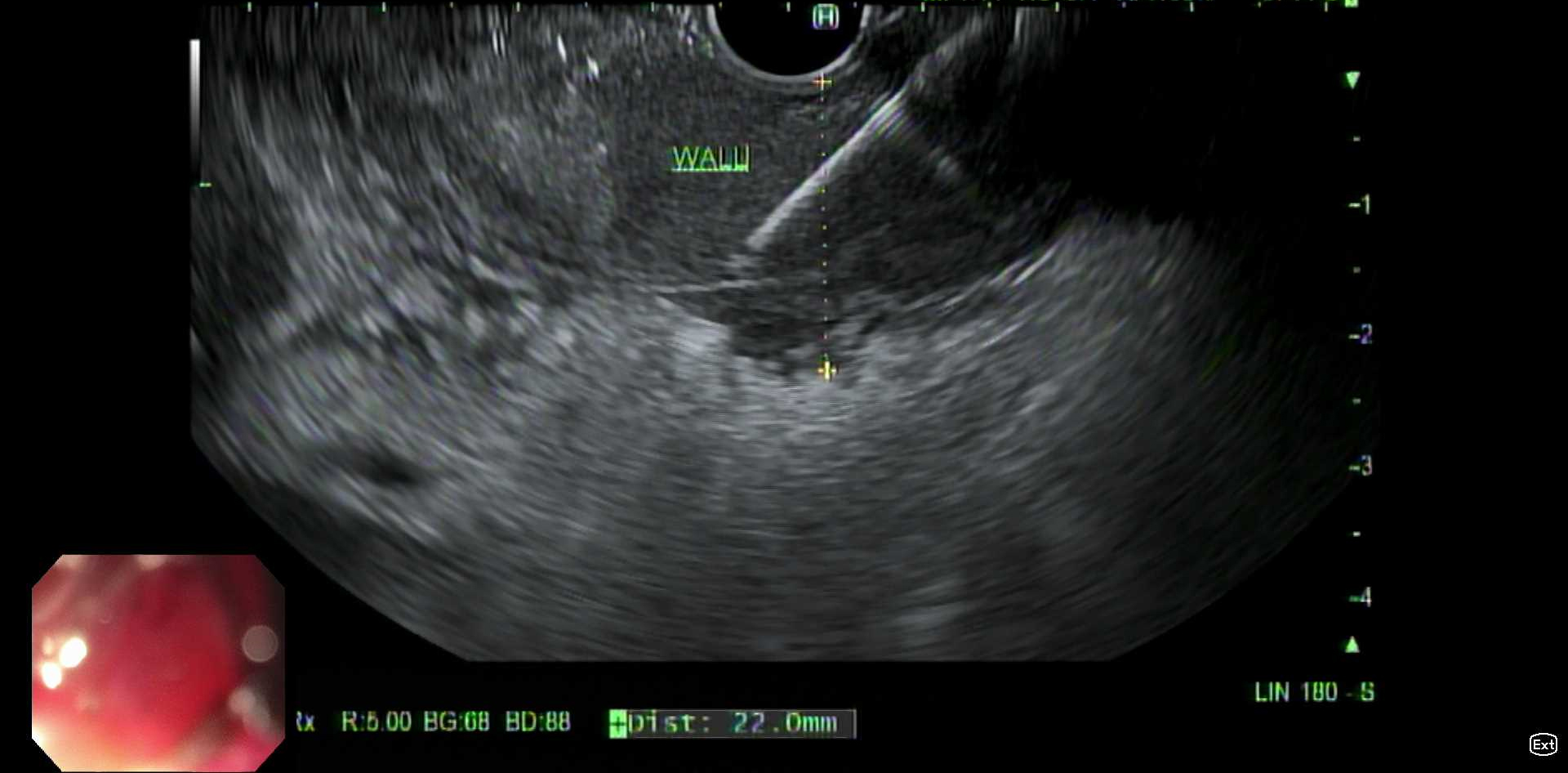
Figure: Endoscopic view of duodenum demonstrating scalloped mucosa and villous atrophy consistent with celiac disease
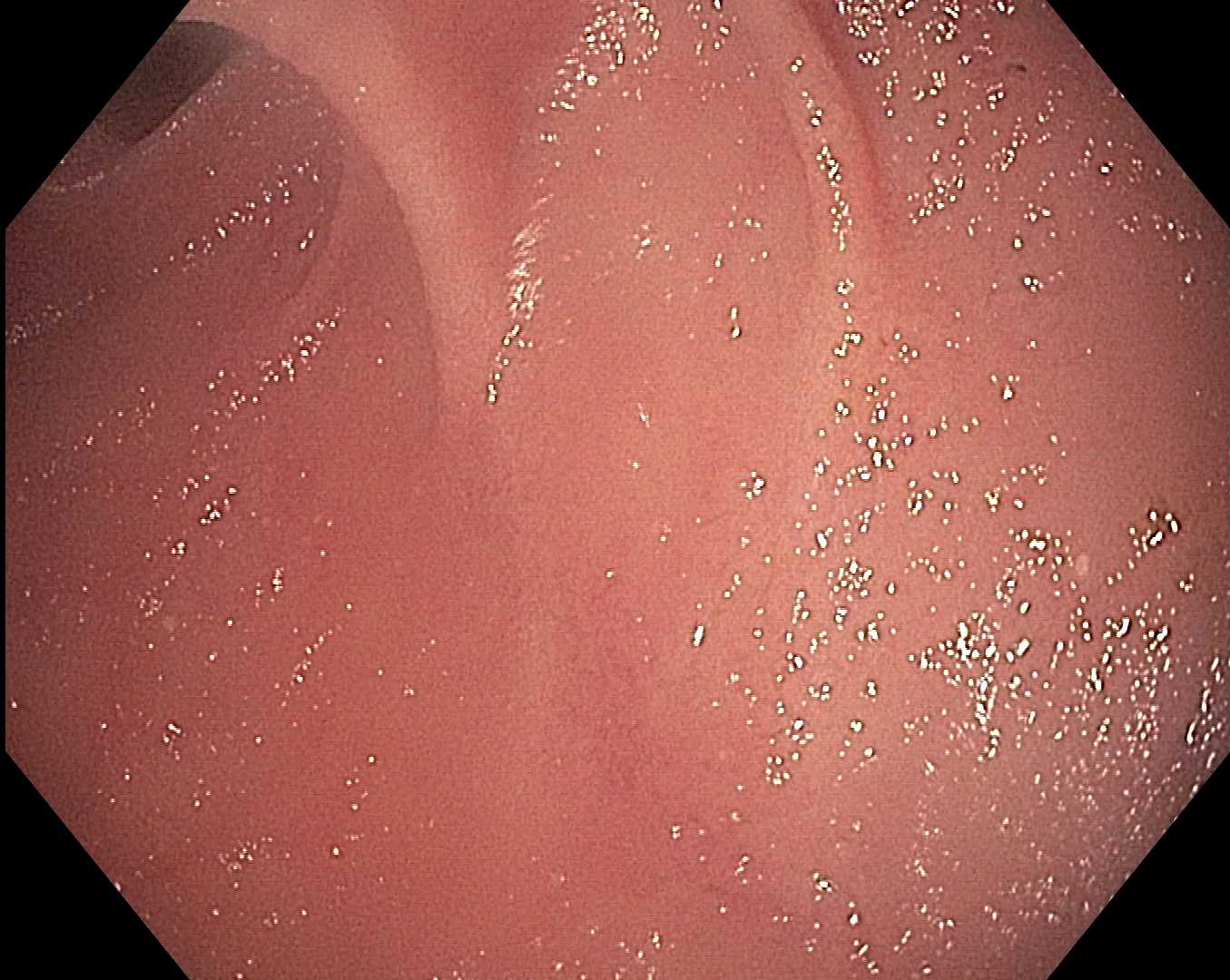
Figure: EUS-guided fine needle biopsy of thickened duodenal wall with complete luminal obstruction
Disclosures:
Kanika Garg indicated no relevant financial relationships.
Agnieszka Maniak indicated no relevant financial relationships.
Thomas Wang indicated no relevant financial relationships.
Christopher Chapman: Boston Scientific – Consultant. Olympus – Consultant. Phathom Pharmaceuticals – Consultant.
Ajaypal Singh: Boston Scientific – Consultant. Creo – Consultant. Olympus – Consultant.
Irving Waxman: Boston Scientific – Consultant. Cook Medical – Consultant. Medtronic – Consultant.
Neal Mehta: Boston Scientific – Consultant. Castle Biosciences – Consultant. ConMed – Consultant. Medtronic – Consultant. Olympus – Consultant.
Kanika Garg, MD, Agnieszka Maniak, MD, Thomas J. Wang, MD, Christopher G. Chapman, MD, Ajaypal Singh, MD, Irving Waxman, MD, FACG, Neal A. Mehta, MD. P1956 - A Rare Complication of Poorly Controlled Celiac Disease: Duodenal Obstruction Secondary to Combined B and T Cell Lymphoma, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Kanika Garg, MD, Agnieszka Maniak, MD, Thomas J. Wang, MD, Christopher G. Chapman, MD, Ajaypal Singh, MD, Irving Waxman, MD, FACG, Neal A. Mehta, MD
Rush University Medical Center, Chicago, IL
Introduction: Celiac disease carries an increased risk of lymphoproliferative disorders, particularly enteropathy-associated T-cell lymphoma (EATL) with a 50-fold increased risk. Diffuse large B-cell lymphoma (DLBCL) occurs at a 3-fold higher rate. Strict adherence to a gluten-free diet is crucial for reducing malignant complications.
Case Description/
Methods: A 62-year-old Spanish-speaking female presented in 2011 with iron deficiency anemia (hemoglobin 10.3 g/dL), abdominal pain, and vitamin D deficiency. Anti-tissue transglutaminase and endomysial antibodies were positive. Esophagogastroduodenoscopy (EGD) demonstrated atrophic mucosa, blunted villi, and luminal narrowing in the duodenum. Pathology confirmed celiac disease (Marsh IIIC). Despite extensive counseling, she was lost to follow-up.
Fourteen years later, without any interval follow-up, she presented with abdominal distension, nausea, and vomiting. She denied adhering to a gluten-free diet. Labs demonstrated worsening anemia (hemoglobin 6.8 g/dL), hypoalbuminemia (albumin 2.4 g/dL), and mild transaminase elevations (AST 55 U/L, ALT 110 U/L). Computed tomography (CT) revealed a 4.3 x 2.4 cm lobulated soft tissue lesion at the hepatic hilum extending distally with circumferential duodenal thickening, resulting in gastric outlet obstruction. CT also showed extensive lymphadenopathy.
EGD and endoscopic ultrasound (EUS) showed complete duodenal bulb obstruction with wall thickening and malignant-appearing porta hepatis lymph nodes (Figure 1, Figure 2). Duodenal and lymph node biopsies were positive for both T and B cell lymphomas. Immunohistochemistry revealed CD20-positive cells and CD3-positive T cells. After further pathologic evaluation and multidisciplinary discussion, she was diagnosed with DLBCL with a subset of T cell population secondary to T cell activation in the setting of long-standing, poorly controlled celiac disease. Bone marrow biopsy confirmed advanced-stage disease.
With conservative management of the obstruction and initiation of inpatient chemotherapy, her duodenal stricture rapidly resolved, allowing her to resume enteral feeding within one week.
Discussion: This case illustrates a rare malignant obstruction from combined DLBCL with T-cell activation in poorly controlled celiac disease. It underscores the importance of dietary adherence and regular follow-up in celiac patients to prevent malignant complications, as well as the diagnostic utility of EUS-guided tissue acquisition in complex lymphoproliferative disorders.

Figure: Endoscopic view of duodenum demonstrating scalloped mucosa and villous atrophy consistent with celiac disease

Figure: EUS-guided fine needle biopsy of thickened duodenal wall with complete luminal obstruction
Disclosures:
Kanika Garg indicated no relevant financial relationships.
Agnieszka Maniak indicated no relevant financial relationships.
Thomas Wang indicated no relevant financial relationships.
Christopher Chapman: Boston Scientific – Consultant. Olympus – Consultant. Phathom Pharmaceuticals – Consultant.
Ajaypal Singh: Boston Scientific – Consultant. Creo – Consultant. Olympus – Consultant.
Irving Waxman: Boston Scientific – Consultant. Cook Medical – Consultant. Medtronic – Consultant.
Neal Mehta: Boston Scientific – Consultant. Castle Biosciences – Consultant. ConMed – Consultant. Medtronic – Consultant. Olympus – Consultant.
Kanika Garg, MD, Agnieszka Maniak, MD, Thomas J. Wang, MD, Christopher G. Chapman, MD, Ajaypal Singh, MD, Irving Waxman, MD, FACG, Neal A. Mehta, MD. P1956 - A Rare Complication of Poorly Controlled Celiac Disease: Duodenal Obstruction Secondary to Combined B and T Cell Lymphoma, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

