Sunday Poster Session
Category: Small Intestine
P1932 - Long-Term Treatment With Once-Weekly Apraglutide Reduces Parenteral Support Dependency and Allows to Reach Enteral Autonomy in Some Patients With Short Bowel Syndrome and Intestinal Failure: The STARS Extend Trial
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- PJ
Palle Jeppesen, MD, PhD
Rigshospitalet, Copenhagen University Hospital
Boston, MA
Presenting Author(s)
Palle Jeppesen, MD, PhD1, Kelly Tappenden, PhD, RD2, Donald Kirby, MD3, Simon Lal, MD, PhD4, Tim Vanuytsel, MD, PhD5, Chang Ming, PhD6, Tomasz Masior, MD6, Mena Boules, MD7, Francisca Joly, MD, PhD8, Kishore Iyer, MBBS, FRCS9
1Rigshospitalet, Copenhagen University Hospital, Boston, MA; 2The University of Utah, Salt Lake City, UT; 3Cleveland Clinic Digestive Disease and Surgery Institute, Cleveland, OH; 4University of Manchester, Manchester, England, United Kingdom; 5Leuven Intestinal Failure and Transplantation Center (LIFT), University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium; 6Ironwood Pharmaceuticals, Inc., Basel, Basel-Stadt, Switzerland; 7Ironwood Pharmaceuticals, Inc., Boston, MA; 8Centre for Intestinal Failure, Department of Gastroenterology and Nutritional Support, Hôpital Beaujon, Clichy, Ile-de-France, France; 9Mount Sinai Medical Center/Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Reducing parenteral support (PS) dependency (fewer days on PS per week) is a key treatment goal in patients (pts) with short bowel syndrome and intestinal failure, with achieving enteral autonomy (PS independence) being the ultimate goal. The long-acting glucagon-like peptide-2 (GLP-2) analog apraglutide (APRA) administered subcutaneously once weekly showed efficacy in reducing PS needs in phase 2 STARS Nutrition and phase 3 STARS trials. STARS Extend (NCT05018286), an open-label, long-term extension (LTE) study, aims to assess the long-term safety and tolerability of APRA, and whether treatment effects are maintained in the long term.
Methods: Eligible pts who completed STARS or STARS Nutrition could opt to continue APRA (APRA/APRA) at dose of 3.5 mg if pt ≥50 kg or 1.4 mg if < 50 kg, or switch from placebo (PBO/APRA), for up to 208 weeks in STARS Extend. The p</span>rimary objective was long-term safety and tolerability; secondary endpoints included changes in PS frequency and pts reaching enteral autonomy at weeks 52, 104, 152, and 208. Baseline (BL) was start of APRA treatment.
Results: As of January 2025, 166 pts were analyzed (APRA/APRA: 119; PBRO/APRA: 47). BL demographics and disease characteristics were generally balanced across groups. At week 52, ≥1 day/week off PS was achieved by 49.1% and 55.3% of APRA/APRA and PBO/APRA pts (Table 1), with small variations in urine volume, fluid intake and body weight (Table 2). Similar results were observed at week 104 (55.4% vs. 50.0%), and for ≥2, ≥3, and ≥4 days/week off PS. 35 pts reached enteral autonomy at least once (24 [20.2%] in APRA/APRA and 11 [23.4%] in PBO/APRA). Of those, 83.3% (20/24) and 90.9% (10/11) maintained continuous EA through last follow-up. Among those achieving enteral autonomy, 24/24 (100%) and 10/11 (90.9%), and 20/24 (83.3%) and 8/11 (72.7%) maintained it for ≥3 and ≥6 months, respectively, in APRA/APRA and PBO/APRA. Body weight at enteral autonomy did not vary from baseline (68.24 kg vs 67.09 kg overall). APRA was well tolerated, with most adverse events being Grade 1/2.
Discussion: Long-term treatment with once-weekly APRA resulted in continued reductions in PS dependency. It led to sustained enteral autonomy, a critical milestone in potentially reducing burden of care and improving QoL, highlighting the potential for ongoing clinical benefit with long-term therapy.
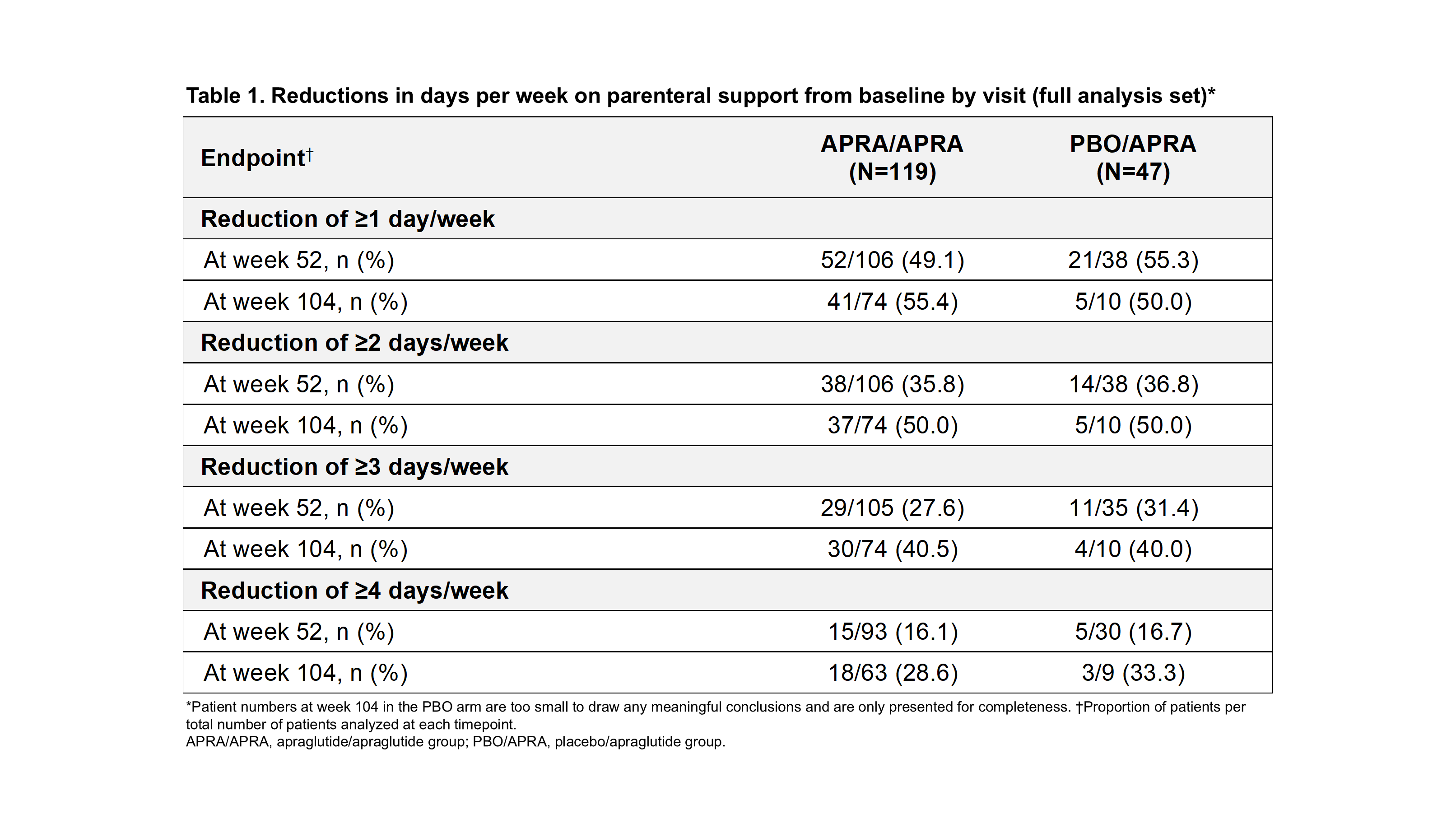
Figure: Table 1
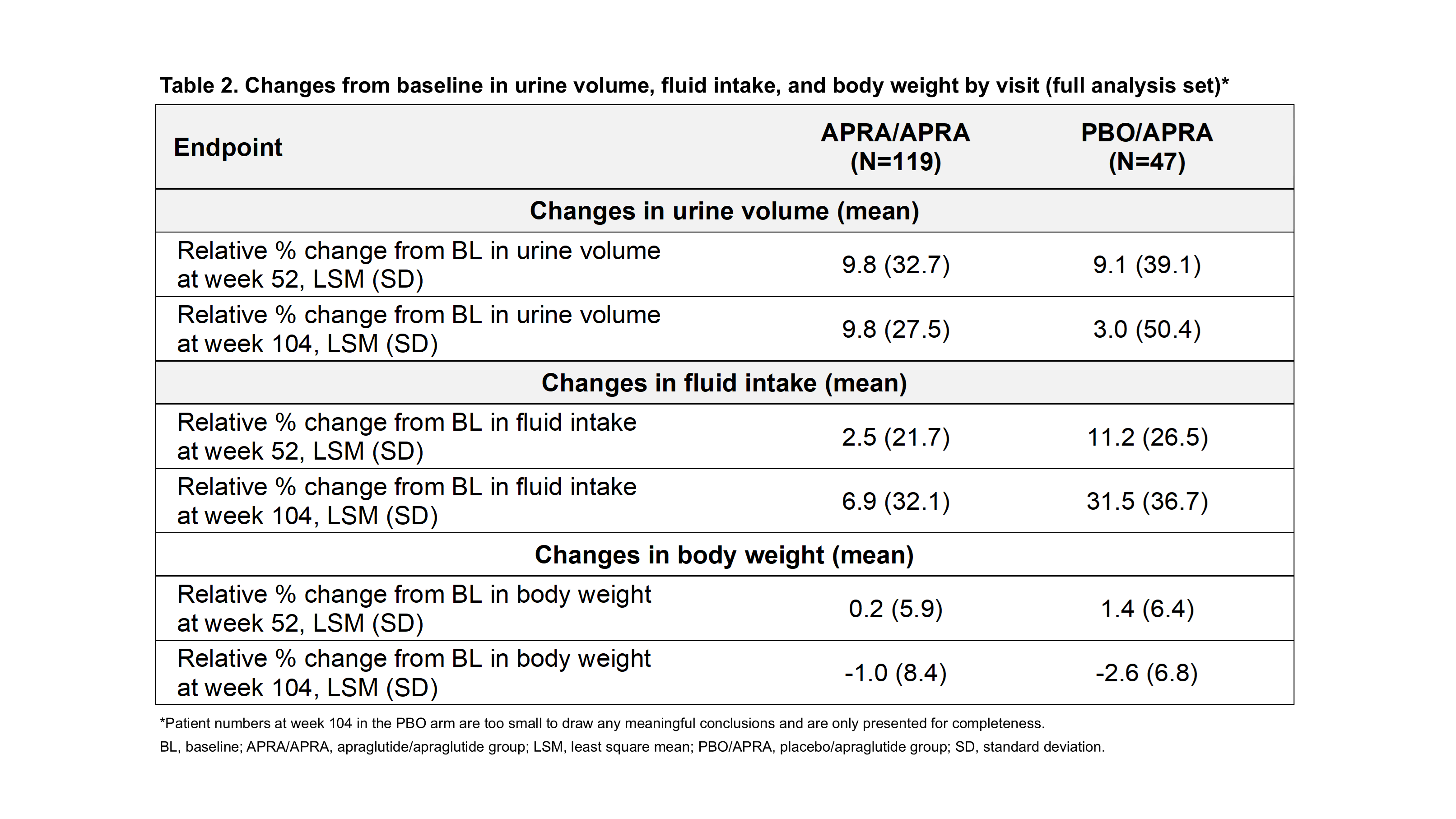
Figure: Table 2
Disclosures:
Palle Jeppesen: Albumedix A/S – Grant/Research Support. ArTara Therapeutics – Grant/Research Support. Bainan Biotech – Grant/Research Support. Baxter – Grant/Research Support. Coloplast A/S – Grant/Research Support. Ferring Pharmaceuticals – Grant/Research Support. Fresenius Kabi – Grant/Research Support. GlyPharma Therapeutic – Grant/Research Support. Hanmi Pharmaceuticals – Grant/Research Support. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Grant/Research Support. Naia Pharmaceuticals – Grant/Research Support. NPS Pharmaceuticals – Grant/Research Support. Protara Therapeutics – Grant/Research Support. Shire – Grant/Research Support. Takeda – Grant/Research Support. The Novo Nordisk Foundation – Grant/Research Support. Therachon – Grant/Research Support. Zealand Pharma A/S – Grant/Research Support.
Kelly Tappenden: Abbott Nutrition Health Institute – Advisory Committee/Board Member. Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Advisory Committee/Board Member. Nutricia North America – Advisory Committee/Board Member. Takeda Pharmaceuticals – Advisory Committee/Board Member.
Donald Kirby: Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Consultant. OWYN – Consultant. Takeda Pharma – Consultant.
Simon Lal: B Braun – Honoraria and/or educational support. Baxter – Grant/Research Support, Honoraria and/or educational support. Fresenius Kabi – Grant/Research Support, Honoraria and/or educational support. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Honoraria and/or educational support. Northsea – Honoraria and/or educational support. Takeda – Grant/Research Support, Honoraria and/or educational support. Zealand – Honoraria and/or educational support.
Tim Vanuytsel: Abbott – Lecturer. Baxter – Consultant, Lecturer. Biocodex – Lecturer. BMS – Consultant. Danone – Grant/Research Support. Dr. Falk Pharma – Consultant, Lecturer. Fresenius Kabi – Lecturer. Hanmi Pharm – Consultant. Ipsen – Lecturer. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Consultant, Grant/Research Support, Lecturer. Menarini – Lecturer. MyHealth – Grant/Research Support, Lecturer. North Sea Therapeutics – Consultant. Remedus – Lecturer. Takeda – Consultant, Grant/Research Support, Lecturer. Truvion – Consultant, Lecturer. Zealand Pharma – Consultant.
Chang Ming: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee.
Tomasz Masior: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee.
Mena Boules: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee, Stock-publicly held company(excluding mutual/index funds).
Francisca Joly: Baxter – Grant/Research Support, Lecturer. BBraun – Lecturer. Carembouche – Consultant, Grant/Research Support. Fresenius Kabi – Lecturer. Hanmi – Consultant. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Consultant, Grant/Research Support. Janssen – Lecturer. Mobile3esolutions – Consultant, Grant/Research Support. NorthSea Therapeutics – Consultant. Takeda – Consultant, Grant/Research Support. Theradial – Consultant. Zealand – Consultant, Grant/Research Support.
Kishore Iyer: Hanmi Pharmaceuticals – Advisor or Review Panel Member. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Advisor or Review Panel Member, Grant/Research Support. Northsea Therapeutics – Advisor or Review Panel Member, Grant/Research Support. Takeda Pharma – Consultant, Grant/Research Support, Speakers Bureau.
Palle Jeppesen, MD, PhD1, Kelly Tappenden, PhD, RD2, Donald Kirby, MD3, Simon Lal, MD, PhD4, Tim Vanuytsel, MD, PhD5, Chang Ming, PhD6, Tomasz Masior, MD6, Mena Boules, MD7, Francisca Joly, MD, PhD8, Kishore Iyer, MBBS, FRCS9. P1932 - Long-Term Treatment With Once-Weekly Apraglutide Reduces Parenteral Support Dependency and Allows to Reach Enteral Autonomy in Some Patients With Short Bowel Syndrome and Intestinal Failure: The STARS Extend Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rigshospitalet, Copenhagen University Hospital, Boston, MA; 2The University of Utah, Salt Lake City, UT; 3Cleveland Clinic Digestive Disease and Surgery Institute, Cleveland, OH; 4University of Manchester, Manchester, England, United Kingdom; 5Leuven Intestinal Failure and Transplantation Center (LIFT), University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium; 6Ironwood Pharmaceuticals, Inc., Basel, Basel-Stadt, Switzerland; 7Ironwood Pharmaceuticals, Inc., Boston, MA; 8Centre for Intestinal Failure, Department of Gastroenterology and Nutritional Support, Hôpital Beaujon, Clichy, Ile-de-France, France; 9Mount Sinai Medical Center/Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Reducing parenteral support (PS) dependency (fewer days on PS per week) is a key treatment goal in patients (pts) with short bowel syndrome and intestinal failure, with achieving enteral autonomy (PS independence) being the ultimate goal. The long-acting glucagon-like peptide-2 (GLP-2) analog apraglutide (APRA) administered subcutaneously once weekly showed efficacy in reducing PS needs in phase 2 STARS Nutrition and phase 3 STARS trials. STARS Extend (NCT05018286), an open-label, long-term extension (LTE) study, aims to assess the long-term safety and tolerability of APRA, and whether treatment effects are maintained in the long term.
Methods: Eligible pts who completed STARS or STARS Nutrition could opt to continue APRA (APRA/APRA) at dose of 3.5 mg if pt ≥50 kg or 1.4 mg if < 50 kg, or switch from placebo (PBO/APRA), for up to 208 weeks in STARS Extend. The p</span>rimary objective was long-term safety and tolerability; secondary endpoints included changes in PS frequency and pts reaching enteral autonomy at weeks 52, 104, 152, and 208. Baseline (BL) was start of APRA treatment.
Results: As of January 2025, 166 pts were analyzed (APRA/APRA: 119; PBRO/APRA: 47). BL demographics and disease characteristics were generally balanced across groups. At week 52, ≥1 day/week off PS was achieved by 49.1% and 55.3% of APRA/APRA and PBO/APRA pts (Table 1), with small variations in urine volume, fluid intake and body weight (Table 2). Similar results were observed at week 104 (55.4% vs. 50.0%), and for ≥2, ≥3, and ≥4 days/week off PS. 35 pts reached enteral autonomy at least once (24 [20.2%] in APRA/APRA and 11 [23.4%] in PBO/APRA). Of those, 83.3% (20/24) and 90.9% (10/11) maintained continuous EA through last follow-up. Among those achieving enteral autonomy, 24/24 (100%) and 10/11 (90.9%), and 20/24 (83.3%) and 8/11 (72.7%) maintained it for ≥3 and ≥6 months, respectively, in APRA/APRA and PBO/APRA. Body weight at enteral autonomy did not vary from baseline (68.24 kg vs 67.09 kg overall). APRA was well tolerated, with most adverse events being Grade 1/2.
Discussion: Long-term treatment with once-weekly APRA resulted in continued reductions in PS dependency. It led to sustained enteral autonomy, a critical milestone in potentially reducing burden of care and improving QoL, highlighting the potential for ongoing clinical benefit with long-term therapy.

Figure: Table 1

Figure: Table 2
Disclosures:
Palle Jeppesen: Albumedix A/S – Grant/Research Support. ArTara Therapeutics – Grant/Research Support. Bainan Biotech – Grant/Research Support. Baxter – Grant/Research Support. Coloplast A/S – Grant/Research Support. Ferring Pharmaceuticals – Grant/Research Support. Fresenius Kabi – Grant/Research Support. GlyPharma Therapeutic – Grant/Research Support. Hanmi Pharmaceuticals – Grant/Research Support. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Grant/Research Support. Naia Pharmaceuticals – Grant/Research Support. NPS Pharmaceuticals – Grant/Research Support. Protara Therapeutics – Grant/Research Support. Shire – Grant/Research Support. Takeda – Grant/Research Support. The Novo Nordisk Foundation – Grant/Research Support. Therachon – Grant/Research Support. Zealand Pharma A/S – Grant/Research Support.
Kelly Tappenden: Abbott Nutrition Health Institute – Advisory Committee/Board Member. Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Advisory Committee/Board Member. Nutricia North America – Advisory Committee/Board Member. Takeda Pharmaceuticals – Advisory Committee/Board Member.
Donald Kirby: Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Consultant. OWYN – Consultant. Takeda Pharma – Consultant.
Simon Lal: B Braun – Honoraria and/or educational support. Baxter – Grant/Research Support, Honoraria and/or educational support. Fresenius Kabi – Grant/Research Support, Honoraria and/or educational support. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Honoraria and/or educational support. Northsea – Honoraria and/or educational support. Takeda – Grant/Research Support, Honoraria and/or educational support. Zealand – Honoraria and/or educational support.
Tim Vanuytsel: Abbott – Lecturer. Baxter – Consultant, Lecturer. Biocodex – Lecturer. BMS – Consultant. Danone – Grant/Research Support. Dr. Falk Pharma – Consultant, Lecturer. Fresenius Kabi – Lecturer. Hanmi Pharm – Consultant. Ipsen – Lecturer. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Consultant, Grant/Research Support, Lecturer. Menarini – Lecturer. MyHealth – Grant/Research Support, Lecturer. North Sea Therapeutics – Consultant. Remedus – Lecturer. Takeda – Consultant, Grant/Research Support, Lecturer. Truvion – Consultant, Lecturer. Zealand Pharma – Consultant.
Chang Ming: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee.
Tomasz Masior: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee.
Mena Boules: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee, Stock-publicly held company(excluding mutual/index funds).
Francisca Joly: Baxter – Grant/Research Support, Lecturer. BBraun – Lecturer. Carembouche – Consultant, Grant/Research Support. Fresenius Kabi – Lecturer. Hanmi – Consultant. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Consultant, Grant/Research Support. Janssen – Lecturer. Mobile3esolutions – Consultant, Grant/Research Support. NorthSea Therapeutics – Consultant. Takeda – Consultant, Grant/Research Support. Theradial – Consultant. Zealand – Consultant, Grant/Research Support.
Kishore Iyer: Hanmi Pharmaceuticals – Advisor or Review Panel Member. Ironwood Pharmaceuticals, Inc. (Previously VectivBio) – Advisor or Review Panel Member, Grant/Research Support. Northsea Therapeutics – Advisor or Review Panel Member, Grant/Research Support. Takeda Pharma – Consultant, Grant/Research Support, Speakers Bureau.
Palle Jeppesen, MD, PhD1, Kelly Tappenden, PhD, RD2, Donald Kirby, MD3, Simon Lal, MD, PhD4, Tim Vanuytsel, MD, PhD5, Chang Ming, PhD6, Tomasz Masior, MD6, Mena Boules, MD7, Francisca Joly, MD, PhD8, Kishore Iyer, MBBS, FRCS9. P1932 - Long-Term Treatment With Once-Weekly Apraglutide Reduces Parenteral Support Dependency and Allows to Reach Enteral Autonomy in Some Patients With Short Bowel Syndrome and Intestinal Failure: The STARS Extend Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
