Sunday Poster Session
Category: Liver
P1751 - Acute Liver Failure Secondary to Risperidone Drug Induced Liver Injury
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MD
Michael DeMarco, DO (he/him/his)
University of Pennsylvania
Philadelphia, PA
Presenting Author(s)
Michael DeMarco, DO1, Hiba Hameed Chagla, MBBCh2, Veena Madhu, MD1, Anila Vasireddy, MD3, Ali Ismail, MD1
1University of Pennsylvania, Philadelphia, PA; 2Penn Medicine, Philadelphia, PA; 3University of Pennsylvania Health System, Philadelphia, PA
Introduction: According to AASLD, the estimated annual incidence of idiosyncratic drug-induced liver injury (DILI) in the general population is 14–19/100,000. Given its relatively infrequent nature, DILI is considered to be a diagnosis of exclusion when assessing liver injury. When accounting for the overall causes of acute liver failure (ALF) though, DILI is the leading cause. This is mainly secondary to acetaminophen toxicity, but additionally due to idiosyncratic DILI. Early recognition of idiosyncratic DILI-related ALF is important, as there is only a 25% likelihood of spontaneous survival. Due to the wide number of drugs associated with idiosyncratic DILI, databases like LiverTox have been developed to help assess the likelihood of any given drug to cause DILI. In this case presentation, we demonstrate what we believe to be a novel presentation of an idiosyncratic DILI caused by Risperidone leading to acute liver failure.
Case Description/
Methods: An 80-year-old female with a PMHx of ESRD on HD, renal cell carcinoma s/p L nephrectomy, and dementia presented to us in the setting of altered mental status. On presentation, she was found to meet criteria for acute liver failure. Her only pertinent history was the recent initiation of risperidone for agitation in the setting of her dementia. GI was promptly consulted, and, as she was not a good transplant candidate, she was not transferred to a transplant facility. She was empirically treated with N-acetylcysteine, and LFTs were monitored for 10 days until they returned near baseline. Work-up was ultimately negative for an alternate cause, including acetaminophen, and DILI from risperidone was determined to be the underlying cause.
Discussion: Risperidone is a widely used antipsychotic with multiple treatment indications, including schizophrenia, bipolar disorder, and often agitation. Risperidone has been associated with liver test abnormalities in up to 30% of patients, usually within the first 8 weeks of treatment, earning it a Likelihood score B on LiverTox. Unfortunately, the mechanism by which risperidone causes a DILI is still unknown. Positively, DILI from risperidone is usually mild and often does not even require dose adjustment or cessation for resolution of injury. To our knowledge, there have been no apparent previously recorded cases of acute liver failure caused by risperidone drug-induced liver injury. We believe this case represents a novel presentation of risperidone’s ability to cause drug-induced liver injury leading to acute liver failure.
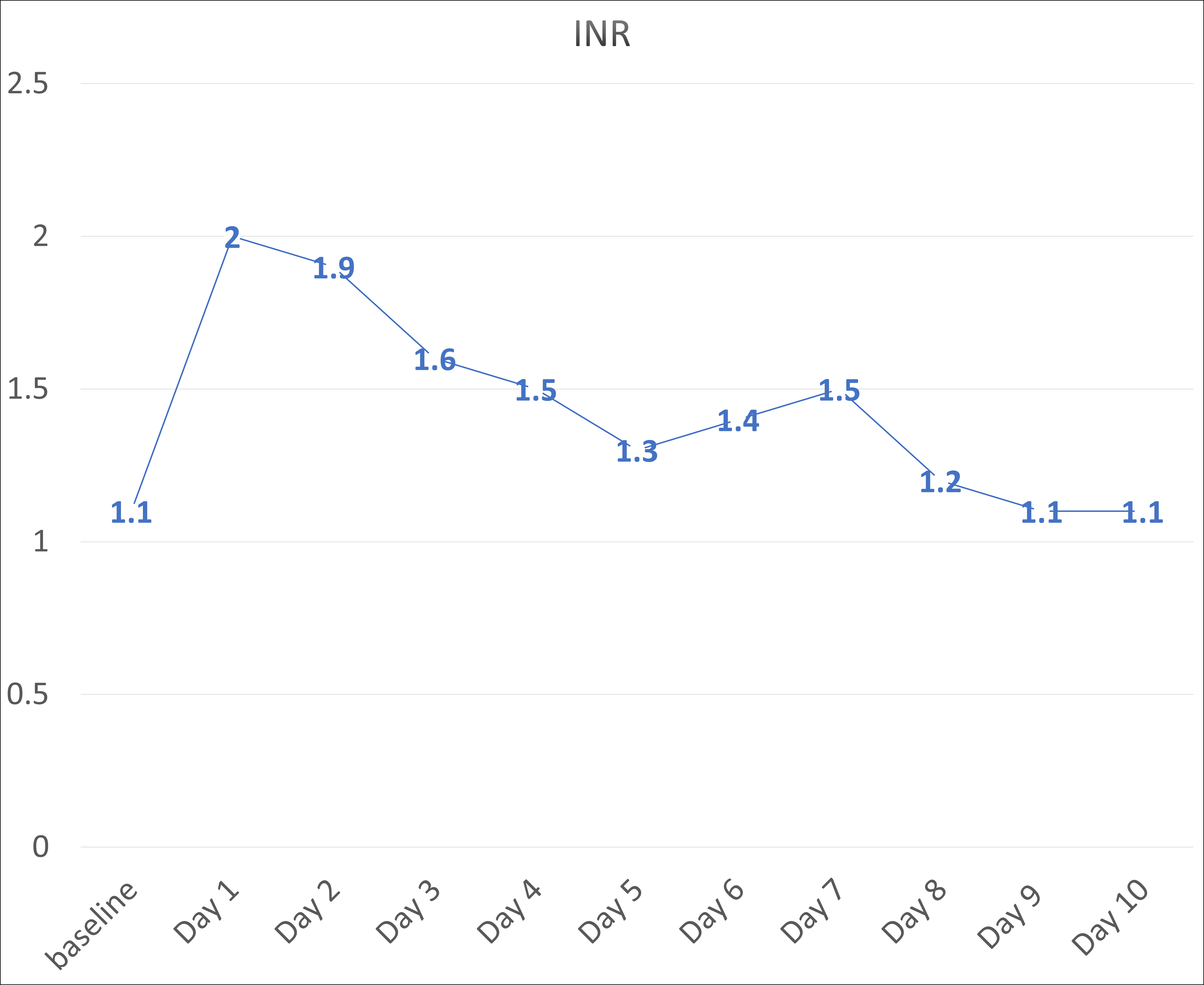
Figure: AST and ALT Trend Over Admission
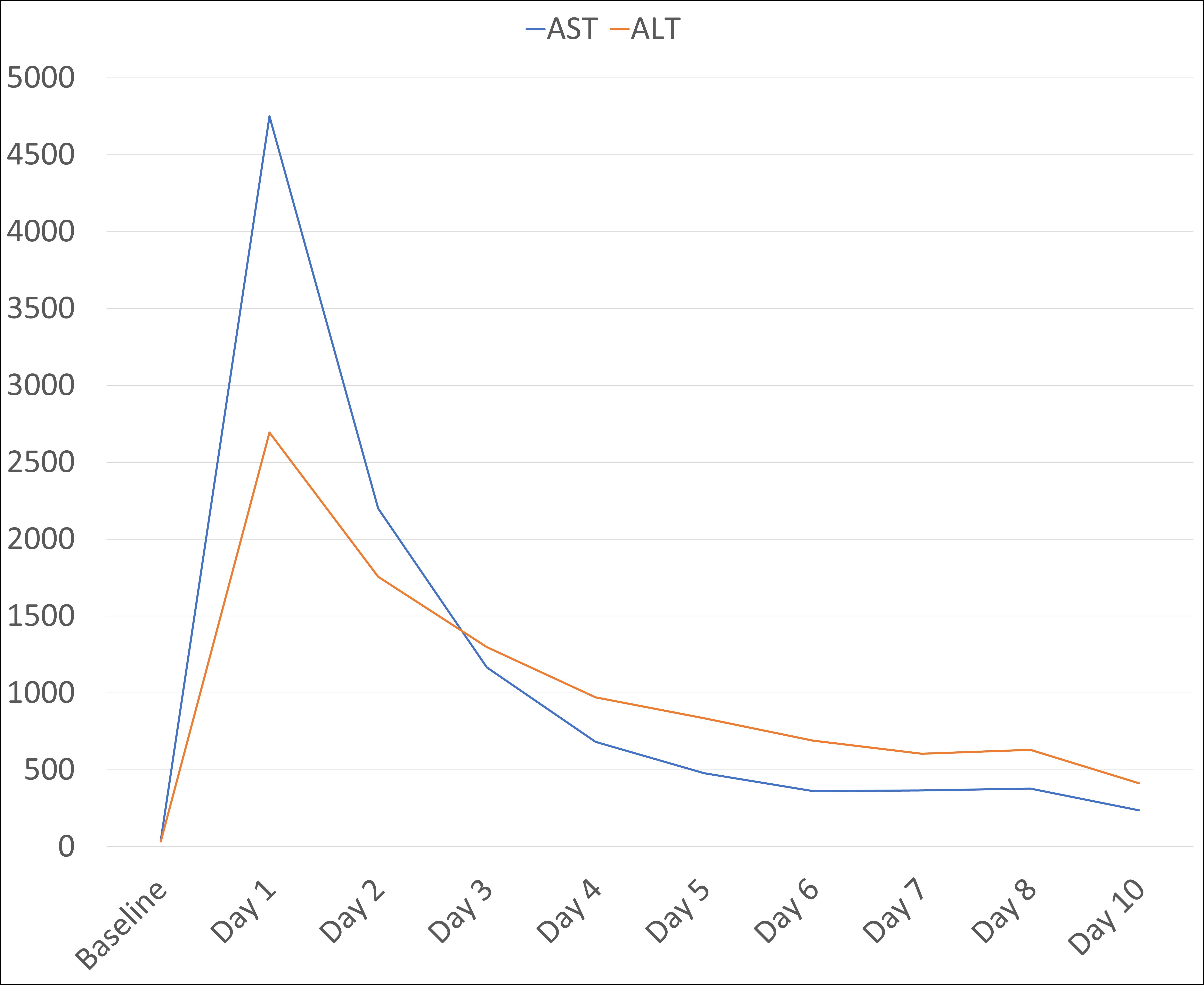
Figure: INR Trend Over Admission
Disclosures:
Michael DeMarco indicated no relevant financial relationships.
Hiba Hameed Chagla indicated no relevant financial relationships.
Veena Madhu indicated no relevant financial relationships.
Anila Vasireddy indicated no relevant financial relationships.
Ali Ismail indicated no relevant financial relationships.
Michael DeMarco, DO1, Hiba Hameed Chagla, MBBCh2, Veena Madhu, MD1, Anila Vasireddy, MD3, Ali Ismail, MD1. P1751 - Acute Liver Failure Secondary to Risperidone Drug Induced Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Pennsylvania, Philadelphia, PA; 2Penn Medicine, Philadelphia, PA; 3University of Pennsylvania Health System, Philadelphia, PA
Introduction: According to AASLD, the estimated annual incidence of idiosyncratic drug-induced liver injury (DILI) in the general population is 14–19/100,000. Given its relatively infrequent nature, DILI is considered to be a diagnosis of exclusion when assessing liver injury. When accounting for the overall causes of acute liver failure (ALF) though, DILI is the leading cause. This is mainly secondary to acetaminophen toxicity, but additionally due to idiosyncratic DILI. Early recognition of idiosyncratic DILI-related ALF is important, as there is only a 25% likelihood of spontaneous survival. Due to the wide number of drugs associated with idiosyncratic DILI, databases like LiverTox have been developed to help assess the likelihood of any given drug to cause DILI. In this case presentation, we demonstrate what we believe to be a novel presentation of an idiosyncratic DILI caused by Risperidone leading to acute liver failure.
Case Description/
Methods: An 80-year-old female with a PMHx of ESRD on HD, renal cell carcinoma s/p L nephrectomy, and dementia presented to us in the setting of altered mental status. On presentation, she was found to meet criteria for acute liver failure. Her only pertinent history was the recent initiation of risperidone for agitation in the setting of her dementia. GI was promptly consulted, and, as she was not a good transplant candidate, she was not transferred to a transplant facility. She was empirically treated with N-acetylcysteine, and LFTs were monitored for 10 days until they returned near baseline. Work-up was ultimately negative for an alternate cause, including acetaminophen, and DILI from risperidone was determined to be the underlying cause.
Discussion: Risperidone is a widely used antipsychotic with multiple treatment indications, including schizophrenia, bipolar disorder, and often agitation. Risperidone has been associated with liver test abnormalities in up to 30% of patients, usually within the first 8 weeks of treatment, earning it a Likelihood score B on LiverTox. Unfortunately, the mechanism by which risperidone causes a DILI is still unknown. Positively, DILI from risperidone is usually mild and often does not even require dose adjustment or cessation for resolution of injury. To our knowledge, there have been no apparent previously recorded cases of acute liver failure caused by risperidone drug-induced liver injury. We believe this case represents a novel presentation of risperidone’s ability to cause drug-induced liver injury leading to acute liver failure.

Figure: AST and ALT Trend Over Admission

Figure: INR Trend Over Admission
Disclosures:
Michael DeMarco indicated no relevant financial relationships.
Hiba Hameed Chagla indicated no relevant financial relationships.
Veena Madhu indicated no relevant financial relationships.
Anila Vasireddy indicated no relevant financial relationships.
Ali Ismail indicated no relevant financial relationships.
Michael DeMarco, DO1, Hiba Hameed Chagla, MBBCh2, Veena Madhu, MD1, Anila Vasireddy, MD3, Ali Ismail, MD1. P1751 - Acute Liver Failure Secondary to Risperidone Drug Induced Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
