Sunday Poster Session
Category: Liver
P1608 - Demographic and Regional Disparities in GLP-1 Receptor Agonist Clinical Trials for Weight Loss: A Comparative Meta-Analysis of US and International RCTs
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Kimberly Ho, MD (she/her/hers)
Brown University
Little Neck, NY
Presenting Author(s)
Kimberly Ho, MD1, Taranika Sarkar Das, MD2, Joao Monteiro, PhD3, Sonali Paul, MD, MS, FACG4, Paige McLean Diaz, MD5
1Brown University, Little Neck, NY; 2The Brooklyn Hospital Center, Brooklyn, NY; 3Brown University, Providence, RI; 4University of Chicago, Chicago, IL; 5Brown Medicine/Brown University Health, Riverside, RI
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) treat obesity, which disproportionately affects racial/ethnic minorities, including 20% of Black adults, 18% Hispanic, and 19% American Native/Indian compared to 16% White adults and 7% Asian Americans in the US (NIH). We conducted a meta-analysis (MA) to assess demographic and regional representation in GLP-1 RA weight-loss randomized controlled trials (RCTs) because we suspect that GLP-1 RA trial generalizability may be limited by relative underrepresentation of key affected patient populations.
Methods: We reviewed 149 English-language RCTs that looked at GLP-1 RAs for weight loss from 2015 to 2025. Random-effects MA assessed demographic and clinical differences in US vs international trials using RRs.
Results: 61 RCTs met inclusion criteria for MA (n=59,425, 45 US, 16 international) (Figure 1). Mean age was 54.7±6.7 years and most were female (95% CI: 46–54%, I² = 98.8%). Whites comprised 69% overall (I² = 99.91%), including 75% in US RCTs and 47% in international RCTs. US RCTs were 4 times more likely to include Whites (4.10 [4.05-4.16]). Hispanic or Latinos made up 24% (I² = 99.84%), with 27% in US vs 11% internationally (RR 1.93 [1.85–2.01]). Blacks were 6% overall (I² = 99.14%), with 6% in US and 7% internationally. US RCTs were 15% less likely to include Blacks (RR 0.85 [0.74-0.97]). Overall, 19% were Asian (I² = 99.97%), with 11% in the US and 42% internationally. RCTs conducted internationally were 7 times more likely to include Asians than those conducted in the US (RR 6.92 [6.86-6.98]). American Indians/Natives (AI/AN) accounted for 2% (I² = 99.91%), with 3% in the US and 1% internationally. RCTS conducted in the US were 15% more likely to include AI/AN (1.15 [0.94-1.36]).
Blacks (6% in this MA vs 20% of US obesity), Hispanic (27% vs 31%), and AI/AN (3% vs 19%) patients were more underrepresented than the overall US obesity prevalence (NIH). Non-Hispanic whites (75% in this MA vs 17% of US obesity) were overrepresented in our study. Asian Americans were properly represented. (Figure 2).
Discussion: These findings reveal substantial underrepresentation of Black, Hispanic, and AI/AN in GLP-1 RA RCTs, especially in the US despite bearing a higher obesity burden. These disparities raise critical concerns about the external validity of GLP-1 RCTs. Inclusive and representative trial enrollment is essential to address existing health disparities across global populations and advance health equity.
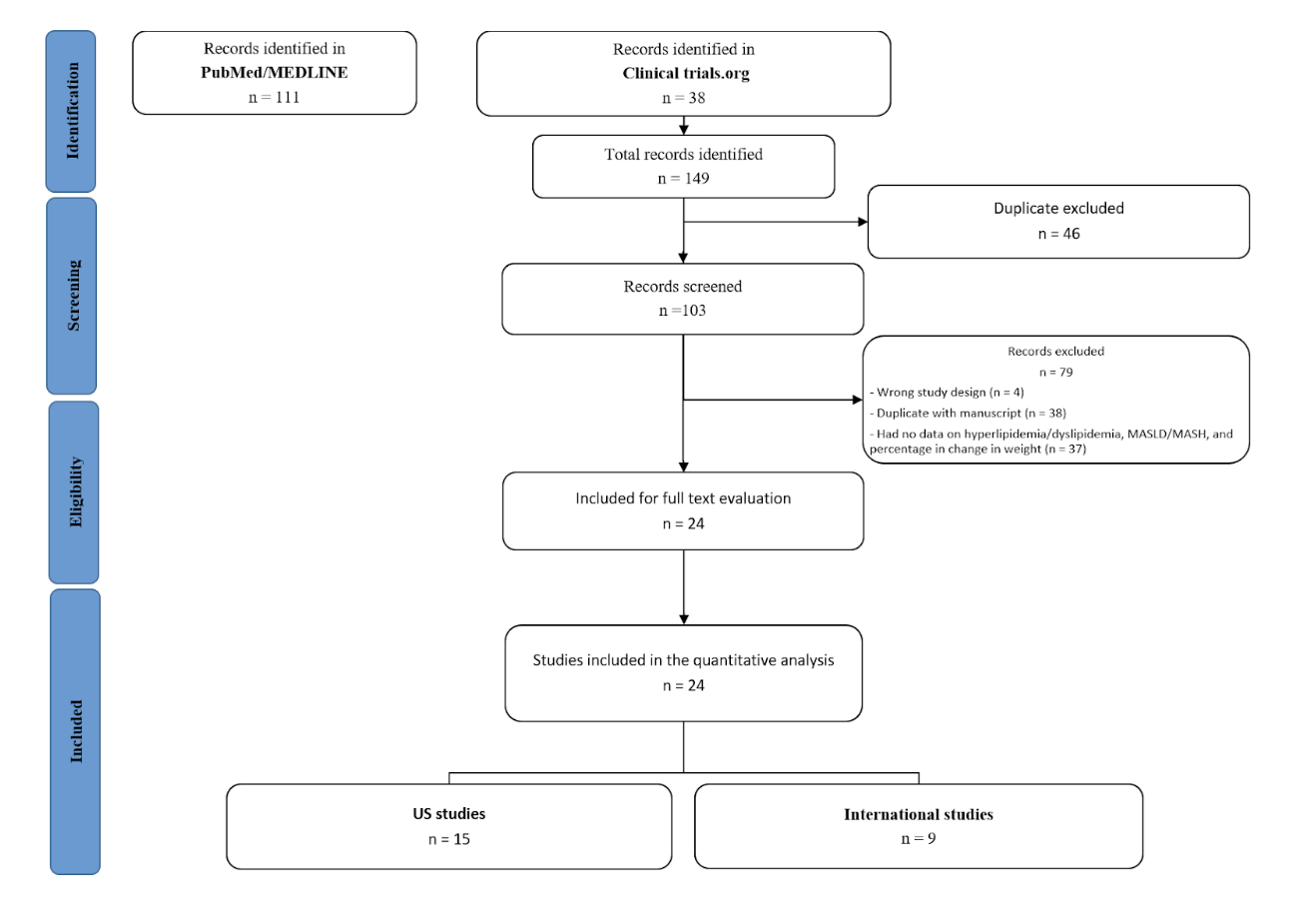
Figure: Figure 1. PRISMA flow chart.
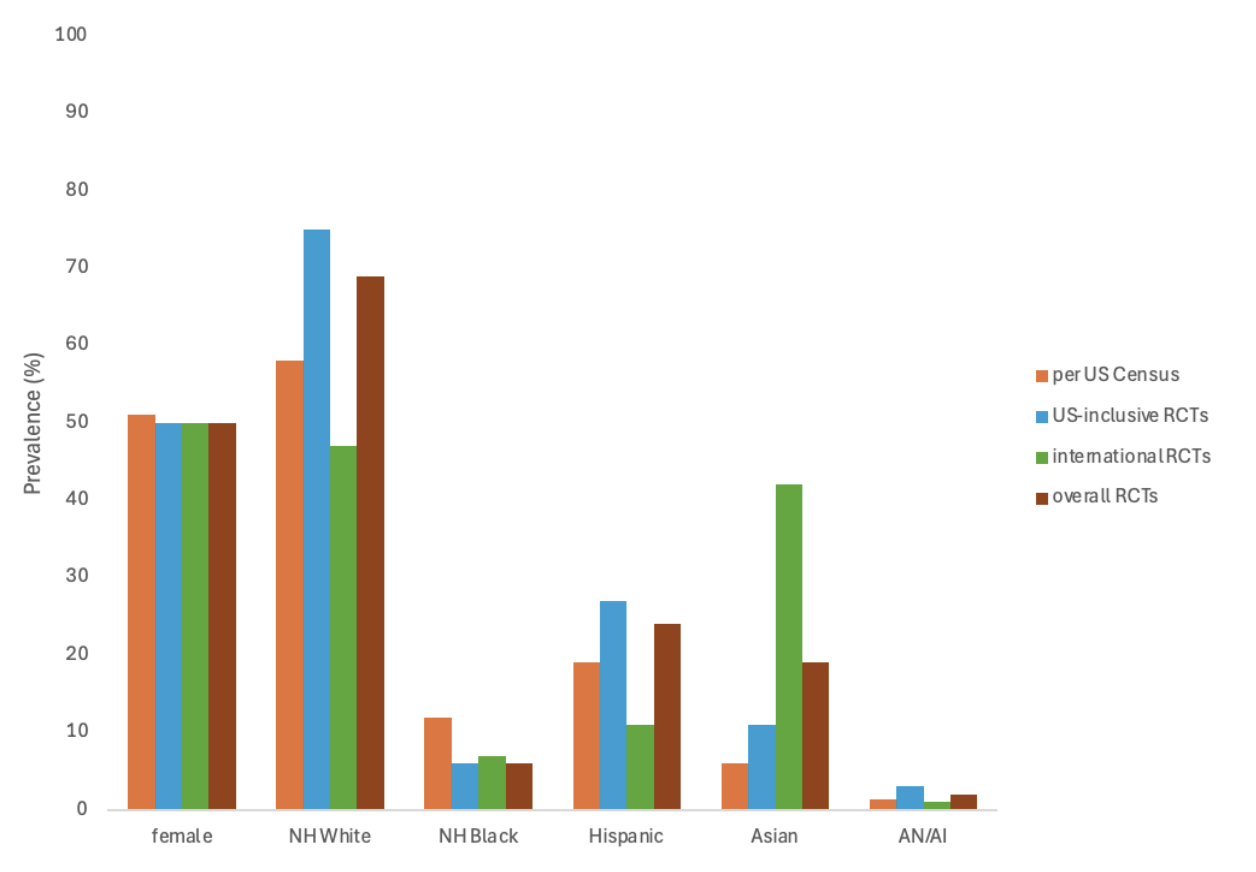
Figure: Demographic data reported in US and international GLP-1 RA clinical trials.
Disclosures:
Kimberly Ho indicated no relevant financial relationships.
Taranika Sarkar Das indicated no relevant financial relationships.
Joao Monteiro indicated no relevant financial relationships.
Sonali Paul: Eli Lilly – Stock-publicly held company(excluding mutual/index funds).
Paige McLean Diaz indicated no relevant financial relationships.
Kimberly Ho, MD1, Taranika Sarkar Das, MD2, Joao Monteiro, PhD3, Sonali Paul, MD, MS, FACG4, Paige McLean Diaz, MD5. P1608 - Demographic and Regional Disparities in GLP-1 Receptor Agonist Clinical Trials for Weight Loss: A Comparative Meta-Analysis of US and International RCTs, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Brown University, Little Neck, NY; 2The Brooklyn Hospital Center, Brooklyn, NY; 3Brown University, Providence, RI; 4University of Chicago, Chicago, IL; 5Brown Medicine/Brown University Health, Riverside, RI
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) treat obesity, which disproportionately affects racial/ethnic minorities, including 20% of Black adults, 18% Hispanic, and 19% American Native/Indian compared to 16% White adults and 7% Asian Americans in the US (NIH). We conducted a meta-analysis (MA) to assess demographic and regional representation in GLP-1 RA weight-loss randomized controlled trials (RCTs) because we suspect that GLP-1 RA trial generalizability may be limited by relative underrepresentation of key affected patient populations.
Methods: We reviewed 149 English-language RCTs that looked at GLP-1 RAs for weight loss from 2015 to 2025. Random-effects MA assessed demographic and clinical differences in US vs international trials using RRs.
Results: 61 RCTs met inclusion criteria for MA (n=59,425, 45 US, 16 international) (Figure 1). Mean age was 54.7±6.7 years and most were female (95% CI: 46–54%, I² = 98.8%). Whites comprised 69% overall (I² = 99.91%), including 75% in US RCTs and 47% in international RCTs. US RCTs were 4 times more likely to include Whites (4.10 [4.05-4.16]). Hispanic or Latinos made up 24% (I² = 99.84%), with 27% in US vs 11% internationally (RR 1.93 [1.85–2.01]). Blacks were 6% overall (I² = 99.14%), with 6% in US and 7% internationally. US RCTs were 15% less likely to include Blacks (RR 0.85 [0.74-0.97]). Overall, 19% were Asian (I² = 99.97%), with 11% in the US and 42% internationally. RCTs conducted internationally were 7 times more likely to include Asians than those conducted in the US (RR 6.92 [6.86-6.98]). American Indians/Natives (AI/AN) accounted for 2% (I² = 99.91%), with 3% in the US and 1% internationally. RCTS conducted in the US were 15% more likely to include AI/AN (1.15 [0.94-1.36]).
Blacks (6% in this MA vs 20% of US obesity), Hispanic (27% vs 31%), and AI/AN (3% vs 19%) patients were more underrepresented than the overall US obesity prevalence (NIH). Non-Hispanic whites (75% in this MA vs 17% of US obesity) were overrepresented in our study. Asian Americans were properly represented. (Figure 2).
Discussion: These findings reveal substantial underrepresentation of Black, Hispanic, and AI/AN in GLP-1 RA RCTs, especially in the US despite bearing a higher obesity burden. These disparities raise critical concerns about the external validity of GLP-1 RCTs. Inclusive and representative trial enrollment is essential to address existing health disparities across global populations and advance health equity.

Figure: Figure 1. PRISMA flow chart.

Figure: Demographic data reported in US and international GLP-1 RA clinical trials.
Disclosures:
Kimberly Ho indicated no relevant financial relationships.
Taranika Sarkar Das indicated no relevant financial relationships.
Joao Monteiro indicated no relevant financial relationships.
Sonali Paul: Eli Lilly – Stock-publicly held company(excluding mutual/index funds).
Paige McLean Diaz indicated no relevant financial relationships.
Kimberly Ho, MD1, Taranika Sarkar Das, MD2, Joao Monteiro, PhD3, Sonali Paul, MD, MS, FACG4, Paige McLean Diaz, MD5. P1608 - Demographic and Regional Disparities in GLP-1 Receptor Agonist Clinical Trials for Weight Loss: A Comparative Meta-Analysis of US and International RCTs, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

