Sunday Poster Session
Category: Liver
P1573 - Drug-Induced Vanishing Bile Duct Syndrome: A Disproportionality Analysis Using the FDA Adverse Event Reporting System (FAERS) Database
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Rohan Karkra, MBBS
Rutgers New Jersey Medical School
Newark, NJ
Presenting Author(s)
Rohan Karkra, MBBS1, Mahinaz Mohsen, MD1, Drew Fletcher, MD1, Ritik M. Goyal, MBBS1, Menna-Allah Elaskandrany, DO2, Kaveh Hajifathalian, MD1, Ahmed Al-Khazraji, MD1
1Rutgers New Jersey Medical School, Newark, NJ; 2Lenox Hill Hospital, Northwell Health, New York, NY
Introduction: Drug-Induced Vanishing Bile Duct Syndrome (DI-VBDS) is a rare and potentially life-threatening adverse event characterized by the progressive destruction and disappearance of intrahepatic bile ducts, leading to cholestasis. The pathogenesis of VBDS is postulated to involve immune-mediated cholangiocyte injury, direct damage by drug metabolites, and sustained exposure to toxic bile salts. This is a real world pharmacovigilance study looking into drug induced vanishing bile duct syndrome.
Methods: The Food and Drug Administration (FDA) maintains the publicly available FDA Adverse Event Reporting System (FAERS) database. As of December 31, 2024, the database contained 30,179,725 reported events. This database was queried to identify all reported cases of ‘Vanishing Bile Duct Syndrome’ (VBDS) secondary to drug use. Events reported worldwide from 2003 to 2024 were identified. All medications implicated in VBDS were identified by their generic names. The database does not confirm whether other causes of VBDS were ruled out. Data on sex, age, country, and mortality was extracted. A disproportionality analysis was conducted, wherein Reporting Odds Ratios (ROR) were calculated.
Results: A total of 899 cases of VBDS were reported to the FAERS from 2003-2024. 893 were characterized as severe, including 166 deaths (18.6%). The most common reported drugs were – Ibuprofen (11.12%; ROR: 32.35 [26.28, 39.83]; p< 0.05), Sulfamethoxazole/Trimethoprim (9.12%; ROR: 63.23 [50.38, 79.37]; p< 0.05), Ciprofloxacin (7.00%; ROR: 37.87 [29.31, 48.92]; p< 0.05), Lamivudine with and without Zidovudine (6.1%; ROR: 59.38 [45.2, 78.02]; p< 0.05), Azithromycin (5.5%; ROR: 59.21 [44.51, 78.78]; p< 0.05), Temozolomide (5.12%; ROR: 66.57 [49.47, 89.59]; p< 0.05), Nevirapine (4.89%; ROR: 117.62 [86.83, 159.33]; p< 0.05), Levofloxacin (4.67%; ROR: 25.06 [18.39, 34.17]; p< 0.05), Acetaminophen (4.67%; ROR: 9.14 [6.7, 12.46]; p< 0.05), Levetiracetam (4.67%; ROR: 19.02 [13.95, 25.93]; p< 0.05), Lamotrigine (4.00%; ROR: 15.64 [11.2, 21.82]; p< 0.05), and Prednisone (3.78%; ROR: 6.72 [4.77, 9.47]; p< 0.05).
Discussion: Ibuprofen was the most reported drug as a cause of DI-VBDS (11% of all reports), while Nevirapine had the highest ROR (117.62) amongst the most frequently reported drugs. High degree of suspicion should be employed when patients present with cholestatic disease after a recent change in medications. Such patients should discontinue these medications when possible.
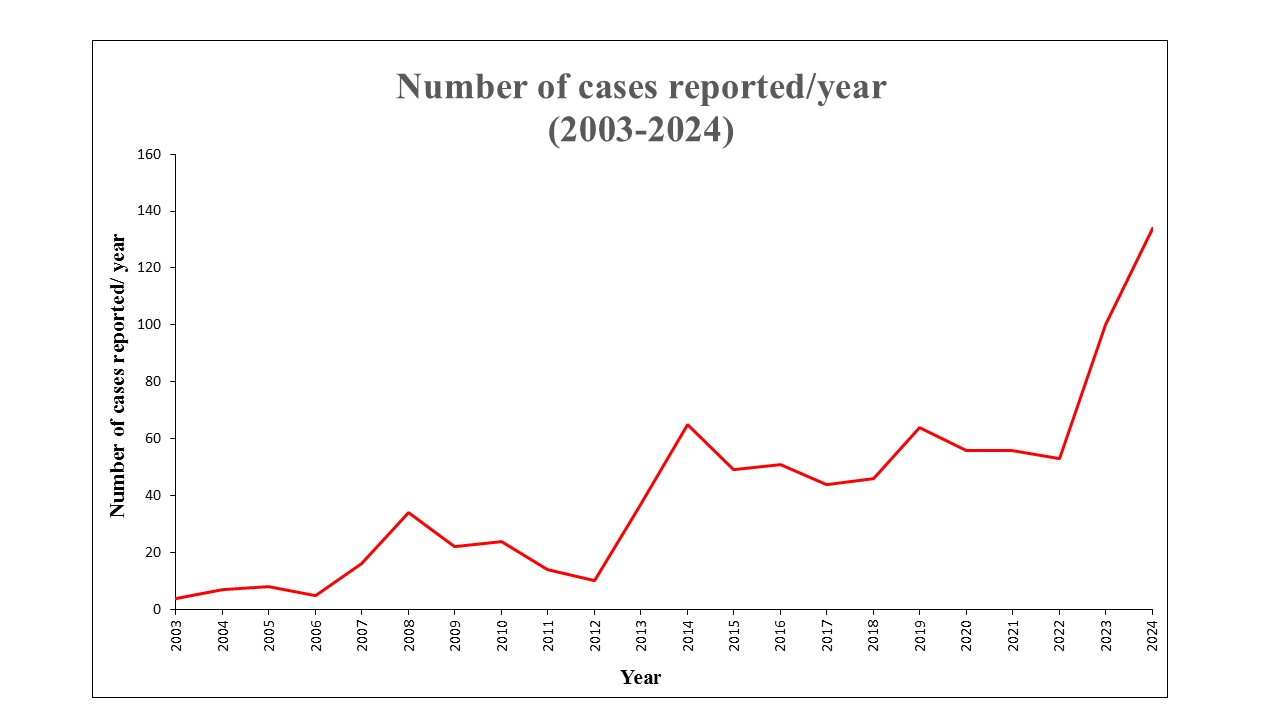
Figure: Number of cases of DI-VBDS reported every year to the FDA-AERS
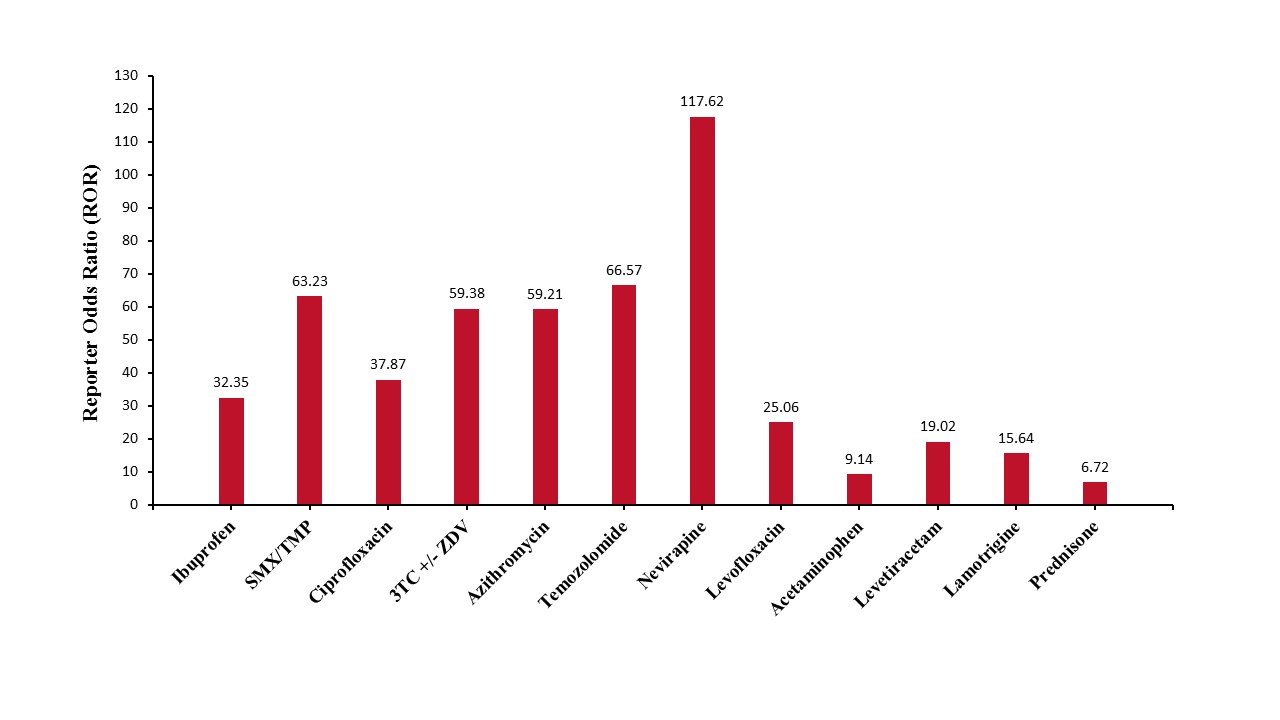
Figure: Reporting Odds Ratios (ROR) for the most frequently reported drugs implicated in DI-VBDS
Disclosures:
Rohan Karkra indicated no relevant financial relationships.
Mahinaz Mohsen indicated no relevant financial relationships.
Drew Fletcher indicated no relevant financial relationships.
Ritik M. Goyal indicated no relevant financial relationships.
Menna-Allah Elaskandrany indicated no relevant financial relationships.
Kaveh Hajifathalian indicated no relevant financial relationships.
Ahmed Al-Khazraji indicated no relevant financial relationships.
Rohan Karkra, MBBS1, Mahinaz Mohsen, MD1, Drew Fletcher, MD1, Ritik M. Goyal, MBBS1, Menna-Allah Elaskandrany, DO2, Kaveh Hajifathalian, MD1, Ahmed Al-Khazraji, MD1. P1573 - Drug-Induced Vanishing Bile Duct Syndrome: A Disproportionality Analysis Using the FDA Adverse Event Reporting System (FAERS) Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rutgers New Jersey Medical School, Newark, NJ; 2Lenox Hill Hospital, Northwell Health, New York, NY
Introduction: Drug-Induced Vanishing Bile Duct Syndrome (DI-VBDS) is a rare and potentially life-threatening adverse event characterized by the progressive destruction and disappearance of intrahepatic bile ducts, leading to cholestasis. The pathogenesis of VBDS is postulated to involve immune-mediated cholangiocyte injury, direct damage by drug metabolites, and sustained exposure to toxic bile salts. This is a real world pharmacovigilance study looking into drug induced vanishing bile duct syndrome.
Methods: The Food and Drug Administration (FDA) maintains the publicly available FDA Adverse Event Reporting System (FAERS) database. As of December 31, 2024, the database contained 30,179,725 reported events. This database was queried to identify all reported cases of ‘Vanishing Bile Duct Syndrome’ (VBDS) secondary to drug use. Events reported worldwide from 2003 to 2024 were identified. All medications implicated in VBDS were identified by their generic names. The database does not confirm whether other causes of VBDS were ruled out. Data on sex, age, country, and mortality was extracted. A disproportionality analysis was conducted, wherein Reporting Odds Ratios (ROR) were calculated.
Results: A total of 899 cases of VBDS were reported to the FAERS from 2003-2024. 893 were characterized as severe, including 166 deaths (18.6%). The most common reported drugs were – Ibuprofen (11.12%; ROR: 32.35 [26.28, 39.83]; p< 0.05), Sulfamethoxazole/Trimethoprim (9.12%; ROR: 63.23 [50.38, 79.37]; p< 0.05), Ciprofloxacin (7.00%; ROR: 37.87 [29.31, 48.92]; p< 0.05), Lamivudine with and without Zidovudine (6.1%; ROR: 59.38 [45.2, 78.02]; p< 0.05), Azithromycin (5.5%; ROR: 59.21 [44.51, 78.78]; p< 0.05), Temozolomide (5.12%; ROR: 66.57 [49.47, 89.59]; p< 0.05), Nevirapine (4.89%; ROR: 117.62 [86.83, 159.33]; p< 0.05), Levofloxacin (4.67%; ROR: 25.06 [18.39, 34.17]; p< 0.05), Acetaminophen (4.67%; ROR: 9.14 [6.7, 12.46]; p< 0.05), Levetiracetam (4.67%; ROR: 19.02 [13.95, 25.93]; p< 0.05), Lamotrigine (4.00%; ROR: 15.64 [11.2, 21.82]; p< 0.05), and Prednisone (3.78%; ROR: 6.72 [4.77, 9.47]; p< 0.05).
Discussion: Ibuprofen was the most reported drug as a cause of DI-VBDS (11% of all reports), while Nevirapine had the highest ROR (117.62) amongst the most frequently reported drugs. High degree of suspicion should be employed when patients present with cholestatic disease after a recent change in medications. Such patients should discontinue these medications when possible.

Figure: Number of cases of DI-VBDS reported every year to the FDA-AERS

Figure: Reporting Odds Ratios (ROR) for the most frequently reported drugs implicated in DI-VBDS
Disclosures:
Rohan Karkra indicated no relevant financial relationships.
Mahinaz Mohsen indicated no relevant financial relationships.
Drew Fletcher indicated no relevant financial relationships.
Ritik M. Goyal indicated no relevant financial relationships.
Menna-Allah Elaskandrany indicated no relevant financial relationships.
Kaveh Hajifathalian indicated no relevant financial relationships.
Ahmed Al-Khazraji indicated no relevant financial relationships.
Rohan Karkra, MBBS1, Mahinaz Mohsen, MD1, Drew Fletcher, MD1, Ritik M. Goyal, MBBS1, Menna-Allah Elaskandrany, DO2, Kaveh Hajifathalian, MD1, Ahmed Al-Khazraji, MD1. P1573 - Drug-Induced Vanishing Bile Duct Syndrome: A Disproportionality Analysis Using the FDA Adverse Event Reporting System (FAERS) Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
