Sunday Poster Session
Category: Liver
P1488 - Severe Immune Checkpoint Inhibitor-Induced Hepatitis: A 6-Month Follow-Up National Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Farhan Azad, DO
INOVA Fairfax Hospital
Fairfax, VA
Presenting Author(s)
Farhan Azad, DO1, Kelly M. Vo, DO2, Amolika Gupta, MD3, Martin Banks, MD4, Adaobi Ahanotu, MD5, Rand Alkalbani, MD1, Marwan S. Abougergi, MD6
1INOVA Fairfax Hospital, Fairfax, VA; 2Walter Reed National Military Medical Center, Bethesda, MD; 3Inova Fairfax Medical Campus, Vienna, VA; 4Bayhealth Medical Center - - Dover, DE, Dover, DE; 5University of Maryland Medical Center, Hyattsville, MD; 6INOVA Fairfax Hospital, Falls Church, VA
Introduction: Immune checkpoint inhibitors (ICI) are revolutionizing the treatment paradigm of many advanced malignancies. A known side effects of this drug class is immune-mediated end organ damage, including immune mediated hepatitis (IMH). Severe IMH (class 3 or 4) is treated in the inpatient setting. No national study has measured the long-term treatment outcomes of severe IMH. We aimed to bridge this gap using the largest national readmission database.
Methods: We used the national readmission database (NRD) to identify adult patients admitted for ICI infusions in the United States from 2016 to 2022. The follow-up period was 6 months. Appropriate ICD-10-CM codes were used to identify diagnoses and procedures. The primary outcome was patients’ demographics. The secondary outcomes were the incidence, readmission rate, mortality rate, of severe IMH as well as its associated rate of acute liver failure and resource utilization.
Results: During the study period, 13,257 patients were hospitalized and received ICI. Of them, 9,014 (68%) were readmitted within 6 months. We identified 41 patients who developed IMH. Patients charactersitics are presented in Table 1. The mean patient age was 48 years. The majority of patients were male, of higher income, and insured mainly by Medicare or private insurance. Most patients were treated at large teaching hospitals in large urban areas. Overall, rising annual incidence of IMH was observed over time (Figure 1), with a noticeable decline in 2020 likely due to the COVID-19 pandemic induced lower hospitalization rates for ICI infusions. The mean time to from infusion to development of IMH was 29 days. The 6-month mortality rate and acute liver failure rates were 4.6% and 5.1%, respectively. The mean length of stay was 16 days with an associated total hospitalization charges of $330,834.
Discussion: Unlike autoimmune hepatitis, severe IMH has a male and middle-age predominance. The incidence of severe IMH is low, indicating that IMH is a low-grade toxicity that resolves with cessation of ICI and corticosteroids for the most part. However, severe IMH occurs within 30 days of infusion and is associated with a substantial risk of mortality, acute liver failure and resource utilization. With dual ICI becoming more common, increased incidence of IMH is expected, and vigilant monitoring of the patients with the demographics we report is recommended for early identification and treatment to avoid detrimental patient outcomes.
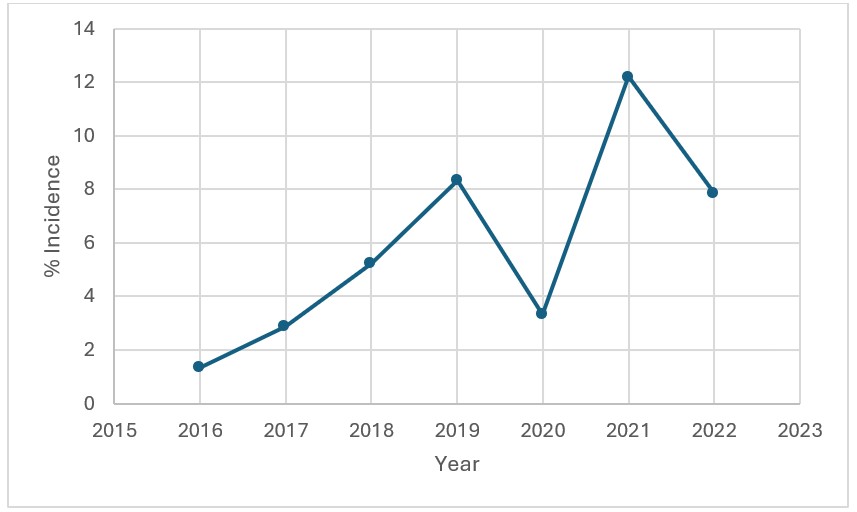
Figure: Increasing incidence of severe immune checkpoint inhibitor-induced hepatitis

Figure: Patient Demographic Characteristics
Disclosures:
Farhan Azad indicated no relevant financial relationships.
Kelly Vo indicated no relevant financial relationships.
Amolika Gupta indicated no relevant financial relationships.
Martin Banks indicated no relevant financial relationships.
Adaobi Ahanotu indicated no relevant financial relationships.
Rand Alkalbani indicated no relevant financial relationships.
Marwan Abougergi indicated no relevant financial relationships.
Farhan Azad, DO1, Kelly M. Vo, DO2, Amolika Gupta, MD3, Martin Banks, MD4, Adaobi Ahanotu, MD5, Rand Alkalbani, MD1, Marwan S. Abougergi, MD6. P1488 - Severe Immune Checkpoint Inhibitor-Induced Hepatitis: A 6-Month Follow-Up National Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1INOVA Fairfax Hospital, Fairfax, VA; 2Walter Reed National Military Medical Center, Bethesda, MD; 3Inova Fairfax Medical Campus, Vienna, VA; 4Bayhealth Medical Center - - Dover, DE, Dover, DE; 5University of Maryland Medical Center, Hyattsville, MD; 6INOVA Fairfax Hospital, Falls Church, VA
Introduction: Immune checkpoint inhibitors (ICI) are revolutionizing the treatment paradigm of many advanced malignancies. A known side effects of this drug class is immune-mediated end organ damage, including immune mediated hepatitis (IMH). Severe IMH (class 3 or 4) is treated in the inpatient setting. No national study has measured the long-term treatment outcomes of severe IMH. We aimed to bridge this gap using the largest national readmission database.
Methods: We used the national readmission database (NRD) to identify adult patients admitted for ICI infusions in the United States from 2016 to 2022. The follow-up period was 6 months. Appropriate ICD-10-CM codes were used to identify diagnoses and procedures. The primary outcome was patients’ demographics. The secondary outcomes were the incidence, readmission rate, mortality rate, of severe IMH as well as its associated rate of acute liver failure and resource utilization.
Results: During the study period, 13,257 patients were hospitalized and received ICI. Of them, 9,014 (68%) were readmitted within 6 months. We identified 41 patients who developed IMH. Patients charactersitics are presented in Table 1. The mean patient age was 48 years. The majority of patients were male, of higher income, and insured mainly by Medicare or private insurance. Most patients were treated at large teaching hospitals in large urban areas. Overall, rising annual incidence of IMH was observed over time (Figure 1), with a noticeable decline in 2020 likely due to the COVID-19 pandemic induced lower hospitalization rates for ICI infusions. The mean time to from infusion to development of IMH was 29 days. The 6-month mortality rate and acute liver failure rates were 4.6% and 5.1%, respectively. The mean length of stay was 16 days with an associated total hospitalization charges of $330,834.
Discussion: Unlike autoimmune hepatitis, severe IMH has a male and middle-age predominance. The incidence of severe IMH is low, indicating that IMH is a low-grade toxicity that resolves with cessation of ICI and corticosteroids for the most part. However, severe IMH occurs within 30 days of infusion and is associated with a substantial risk of mortality, acute liver failure and resource utilization. With dual ICI becoming more common, increased incidence of IMH is expected, and vigilant monitoring of the patients with the demographics we report is recommended for early identification and treatment to avoid detrimental patient outcomes.

Figure: Increasing incidence of severe immune checkpoint inhibitor-induced hepatitis

Figure: Patient Demographic Characteristics
Disclosures:
Farhan Azad indicated no relevant financial relationships.
Kelly Vo indicated no relevant financial relationships.
Amolika Gupta indicated no relevant financial relationships.
Martin Banks indicated no relevant financial relationships.
Adaobi Ahanotu indicated no relevant financial relationships.
Rand Alkalbani indicated no relevant financial relationships.
Marwan Abougergi indicated no relevant financial relationships.
Farhan Azad, DO1, Kelly M. Vo, DO2, Amolika Gupta, MD3, Martin Banks, MD4, Adaobi Ahanotu, MD5, Rand Alkalbani, MD1, Marwan S. Abougergi, MD6. P1488 - Severe Immune Checkpoint Inhibitor-Induced Hepatitis: A 6-Month Follow-Up National Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
