Sunday Poster Session
Category: Interventional Endoscopy
P1438 - Brain Fog in Patients With Juvenile Polyposis Syndrome: Clinical Pearls
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Anusha Agarwal, MD (she/her/hers)
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
Anusha Agarwal, MD1, Denisse Rubio-Cruz, MD1, Mohamed Tausif-Siddiqui, MD1, Kyungran Justina Cho, MD, PhD2, Amit Bhatt, MD1
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic Foundation, Pepper Pike, OH
Introduction: Sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, has emerged as an alternative treatment modality in Juvenile Polyposis Syndrome (JPS). We uniquely describe the development of neurologic symptoms potentially due to sirolimus use in two patients with JPS.
Case Description/
Methods: The first patient is a 28-year-old-male with JPS, Cowden's syndrome, duodenal cancer status post Whipple’s procedure, and hypothyroidism who was initiated on sirolimus therapy in February 2024. One month after the initial sirolimus starting dose of 1 mg daily, patient developed leukopenia. One month after dose reduction to 0.5 mg daily, leukopenia resolved, but two months thereafter, patient developed brain fog, fatigue, intermittent confusion, and mild anhedonia. Sirolimus trough level at time of neurologic symptoms was 1.9 ng/mL (normal range 3 – 20 ng/mL). Endoscopy revealed significant reduction in the gastric and small bowel polyposis after 6-months of sirolimus therapy (Figure 1). Bloodwork revealed no electrolyte abnormalities and well-managed hypothyroidism on levothyroxine. Patient subsequently stopped sirolimus in July 2024, after which he had resolution of his neurologic symptoms.
The second patient is a 35-year-old-male with JPS and postural orthostatic tachycardia syndrome on sirolimus therapy since 2021 with significant endoscopic reduction in initially observed massive gastric polyposis. Patient was noted to have tolerated sirolimus until July 2024, when he developed worsening fatigue and brain fog. Sirolimus trough level in December 2024 was 2.4 ng/mL. Patient is without any electrolyte abnormalities and is currently not on any other notable medications. Sirolimus was discontinued in May 2025 with referral to neurology for work-up with an MRI of his brain.
Discussion: These cases highlight potential neurotoxicity that arises from sirolimus use. Side effects of sirolimus include dyslipidemia, cytopenia, nephrotoxicity, and to a lesser extent, neurotoxicity. Neurotoxicity mostly includes cognitive impairment or posterior reversible encephalopathy syndrome (PRES) which commonly manifests as brain fog. Sirolimus-induced neurotoxicity is scarcely reported, with most cases being related to dose-independent toxicity. As such, we encourage clinicians to be aware of neurotoxicity in patients with JPS managed with sirolimus to allow for timely recognition and appropriate management with serial lab monitoring, imaging, discontinuation of therapy, and co-management with neurology.
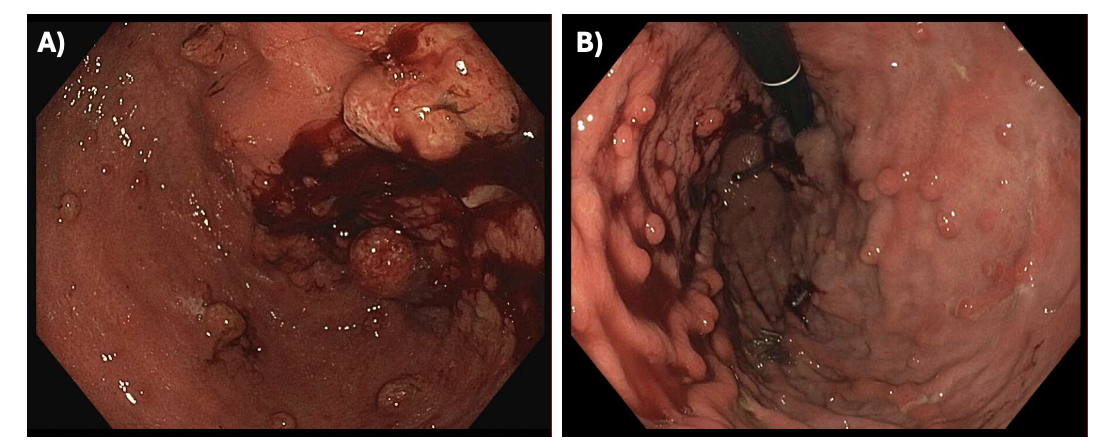
Figure: Figure 1: Endoscopic Findings in Patient with JPS on Sirolimus Therapy. A) Initial endoscopy of gastric body completed in February 2024 prior to start of sirolimus. B) Endoscopic findings showing significant reduction in gastric polyps in August 2024 after patient completed 6 months of sirolimus therapy.
Disclosures:
Anusha Agarwal indicated no relevant financial relationships.
Denisse Rubio-Cruz indicated no relevant financial relationships.
Mohamed Tausif-Siddiqui indicated no relevant financial relationships.
Kyungran Justina Cho indicated no relevant financial relationships.
Amit Bhatt indicated no relevant financial relationships.
Anusha Agarwal, MD1, Denisse Rubio-Cruz, MD1, Mohamed Tausif-Siddiqui, MD1, Kyungran Justina Cho, MD, PhD2, Amit Bhatt, MD1. P1438 - Brain Fog in Patients With Juvenile Polyposis Syndrome: Clinical Pearls, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic Foundation, Pepper Pike, OH
Introduction: Sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, has emerged as an alternative treatment modality in Juvenile Polyposis Syndrome (JPS). We uniquely describe the development of neurologic symptoms potentially due to sirolimus use in two patients with JPS.
Case Description/
Methods: The first patient is a 28-year-old-male with JPS, Cowden's syndrome, duodenal cancer status post Whipple’s procedure, and hypothyroidism who was initiated on sirolimus therapy in February 2024. One month after the initial sirolimus starting dose of 1 mg daily, patient developed leukopenia. One month after dose reduction to 0.5 mg daily, leukopenia resolved, but two months thereafter, patient developed brain fog, fatigue, intermittent confusion, and mild anhedonia. Sirolimus trough level at time of neurologic symptoms was 1.9 ng/mL (normal range 3 – 20 ng/mL). Endoscopy revealed significant reduction in the gastric and small bowel polyposis after 6-months of sirolimus therapy (Figure 1). Bloodwork revealed no electrolyte abnormalities and well-managed hypothyroidism on levothyroxine. Patient subsequently stopped sirolimus in July 2024, after which he had resolution of his neurologic symptoms.
The second patient is a 35-year-old-male with JPS and postural orthostatic tachycardia syndrome on sirolimus therapy since 2021 with significant endoscopic reduction in initially observed massive gastric polyposis. Patient was noted to have tolerated sirolimus until July 2024, when he developed worsening fatigue and brain fog. Sirolimus trough level in December 2024 was 2.4 ng/mL. Patient is without any electrolyte abnormalities and is currently not on any other notable medications. Sirolimus was discontinued in May 2025 with referral to neurology for work-up with an MRI of his brain.
Discussion: These cases highlight potential neurotoxicity that arises from sirolimus use. Side effects of sirolimus include dyslipidemia, cytopenia, nephrotoxicity, and to a lesser extent, neurotoxicity. Neurotoxicity mostly includes cognitive impairment or posterior reversible encephalopathy syndrome (PRES) which commonly manifests as brain fog. Sirolimus-induced neurotoxicity is scarcely reported, with most cases being related to dose-independent toxicity. As such, we encourage clinicians to be aware of neurotoxicity in patients with JPS managed with sirolimus to allow for timely recognition and appropriate management with serial lab monitoring, imaging, discontinuation of therapy, and co-management with neurology.

Figure: Figure 1: Endoscopic Findings in Patient with JPS on Sirolimus Therapy. A) Initial endoscopy of gastric body completed in February 2024 prior to start of sirolimus. B) Endoscopic findings showing significant reduction in gastric polyps in August 2024 after patient completed 6 months of sirolimus therapy.
Disclosures:
Anusha Agarwal indicated no relevant financial relationships.
Denisse Rubio-Cruz indicated no relevant financial relationships.
Mohamed Tausif-Siddiqui indicated no relevant financial relationships.
Kyungran Justina Cho indicated no relevant financial relationships.
Amit Bhatt indicated no relevant financial relationships.
Anusha Agarwal, MD1, Denisse Rubio-Cruz, MD1, Mohamed Tausif-Siddiqui, MD1, Kyungran Justina Cho, MD, PhD2, Amit Bhatt, MD1. P1438 - Brain Fog in Patients With Juvenile Polyposis Syndrome: Clinical Pearls, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
