Sunday Poster Session
Category: Interventional Endoscopy
P1408 - The Safety of Peri-ERCP Rectal Prophylactic Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Patients With Advanced Chronic Kidney Disease (GFR < 30)
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MA
Mohammed Abusuliman, MD
Henry Ford Hospital
Dearborn, MI
Presenting Author(s)
Mohammed Abusuliman, MD1, Azizullah Beran, MD2, Khaled Elfert, MBChB, MRCP3, Amr Abusuliman, MD4, Mohamed Eldesouki, MD5, Mohammed Y. Youssef, MD6, Hazem Abosheaishaa, MD7, Jonathan A. Montrose, DO8, Anas A. Elsaka, MD9, Mona A. Ali, MD9, Ahmed Salem, MD10, Mark Gromski, MD2, Sherif E. Elhanafi, MD11
1Henry Ford Hospital, Detroit, MI; 2Indiana University School of Medicine, Indianapolis, IN; 3West Virginia University School of Medicine, Morgantown, WV; 4Tanta University, Qutour, Al Gharbiyah, Egypt; 5Saint Michael's Medical Center, New York Medical College, Newark, NJ; 6Hunt Regional Medical Center, Greenville, TX; 7Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY; 8Henry Ford Health, Detroit, MI; 9Mansoura University, Mansoura, Ad Daqahliyah, Egypt; 10Maimonides Medical Center, Brooklyn, NY; 11Texas Tech University Health Sciences Center, El Paso, TX
Introduction: Post-Endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis (PEP) is a common and serious complication of ERCP. Rectal nonsteroidal anti-inflammatory drugs (NSAIDs) like indomethacin and diclofenac are widely used for prophylaxis due to their anti-inflammatory effects. However, their use in patients with advanced chronic kidney disease (CKD) raises concerns about nephrotoxicity with a risk of acute kidney injury (AKI). Real-world data on the safety of NSAID use in CKD stages 4–5 in the context of endoscopic procedures is limited. We aimed to assess and weigh the renal safety with the incidence of AKI following NSAIDs use vs the prophylactic benefit of protecting against PEP in advanced CKD patients.
Methods: Utilizing the TriNetX US Collaborative Network, we identified CKD stage 4 and 5 patients (with GFR < 30, excluding those on dialysis/renal replacement therapy) who underwent ERCP and received rectal NSAIDs prophylaxis during the procedure, and compared them to another matched group of CKD patients who underwent ERCP and did not receive prophylaxis. The primary outcome was the development of AKI following the ERCP. Secondary outcomes included PEP, gastrointestinal bleeding (GIB), ICU admission, acute necrotizing pancreatitis (ANP), sepsis, intubation, and mortality. We matched the groups based on baseline characteristics, co-morbidities, and pertinent medications that increase the risk of kidney injury (including diuretics, ACEi, ARBs, etc) to rule out potential confounders.
Results: A total of 1,490 patients with CKD stages 4 or 5 received NSAIDs and 3,773 patients who did not were identified before propensity score matching, all of whom underwent ERCP. After matching, each group included 1,470 patients with comparable baseline characteristics. (Table 1).
There was no significant difference in the incidence of AKI between both groups (13% vs. 12%, p = 0.243). However, PEP was less common in the NSAID group (8% vs. 10%, p = 0.027). There was no difference in rates of GIB, intubation, sepsis, or mortality. Notably, ANP occurred only in the non-NSAIDs group (0% vs. 0.7%, p = 0.002). (Table 2)
Discussion: In patients with advanced CKD (GFR< 30) undergoing ERCP, NSAIDs were not linked to higher risks of AKI, GI bleeding, or mortality; they were associated with lower PEP risk. These findings suggest that, with appropriate patient selection, NSAIDs may be safely used in this high risk patient population for single doses.
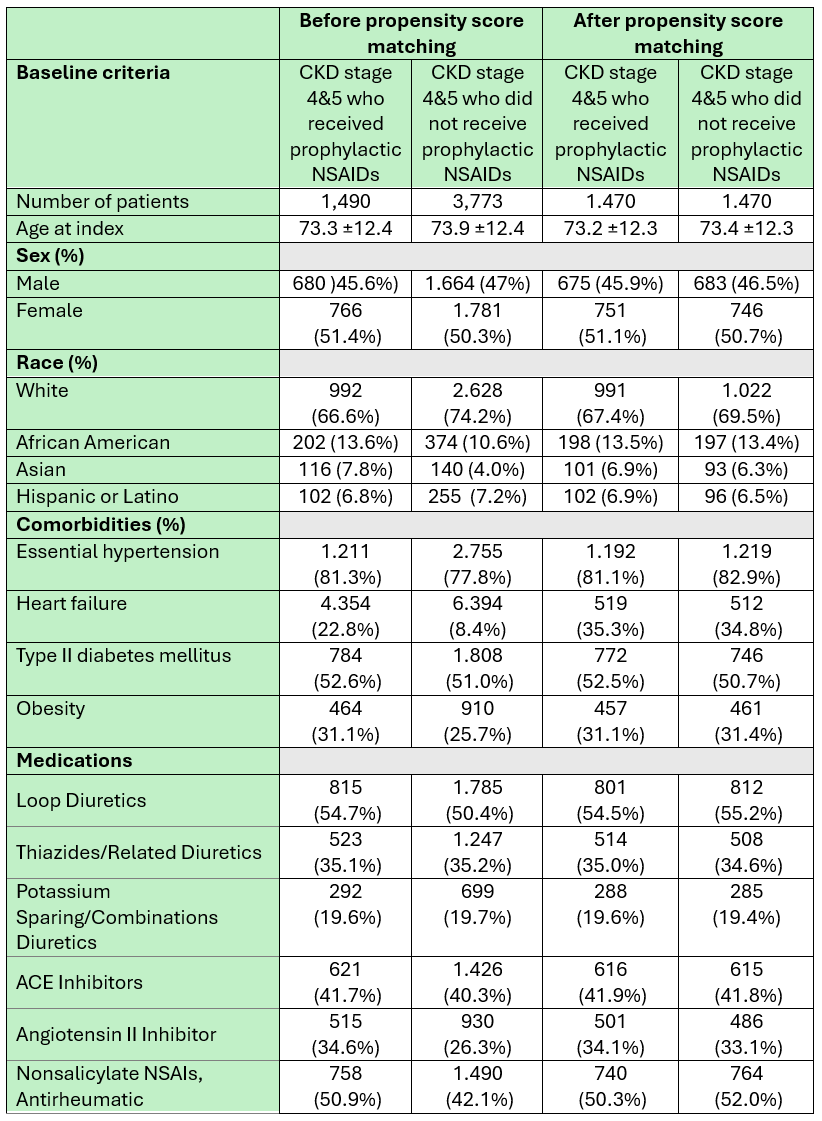
Figure: Table 1: baseline characteristics, co-morbidities, and pertinent medications before and after propensity score matching
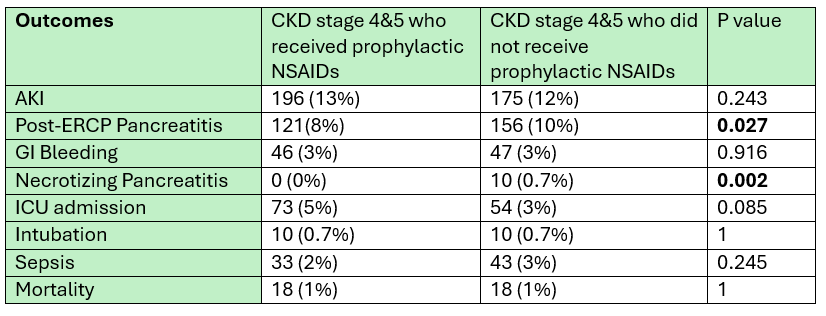
Figure: Table 2: outcomes
Disclosures:
Mohammed Abusuliman indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Amr Abusuliman indicated no relevant financial relationships.
Mohamed Eldesouki indicated no relevant financial relationships.
Mohammed Y. Youssef indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Jonathan Montrose indicated no relevant financial relationships.
Anas Elsaka indicated no relevant financial relationships.
Mona Ali indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Mark Gromski: Grant/Research/Clinical Trial Support – Allurion; Cook Medical; Fractyl. Consultant/Advisory Boards – Ambu; Boston Scientific; Cook Medical – Advisory Committee/Board Member, Grant/Research Support.
Sherif Elhanafi indicated no relevant financial relationships.
Mohammed Abusuliman, MD1, Azizullah Beran, MD2, Khaled Elfert, MBChB, MRCP3, Amr Abusuliman, MD4, Mohamed Eldesouki, MD5, Mohammed Y. Youssef, MD6, Hazem Abosheaishaa, MD7, Jonathan A. Montrose, DO8, Anas A. Elsaka, MD9, Mona A. Ali, MD9, Ahmed Salem, MD10, Mark Gromski, MD2, Sherif E. Elhanafi, MD11. P1408 - The Safety of Peri-ERCP Rectal Prophylactic Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Patients With Advanced Chronic Kidney Disease (GFR < 30), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Henry Ford Hospital, Detroit, MI; 2Indiana University School of Medicine, Indianapolis, IN; 3West Virginia University School of Medicine, Morgantown, WV; 4Tanta University, Qutour, Al Gharbiyah, Egypt; 5Saint Michael's Medical Center, New York Medical College, Newark, NJ; 6Hunt Regional Medical Center, Greenville, TX; 7Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY; 8Henry Ford Health, Detroit, MI; 9Mansoura University, Mansoura, Ad Daqahliyah, Egypt; 10Maimonides Medical Center, Brooklyn, NY; 11Texas Tech University Health Sciences Center, El Paso, TX
Introduction: Post-Endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis (PEP) is a common and serious complication of ERCP. Rectal nonsteroidal anti-inflammatory drugs (NSAIDs) like indomethacin and diclofenac are widely used for prophylaxis due to their anti-inflammatory effects. However, their use in patients with advanced chronic kidney disease (CKD) raises concerns about nephrotoxicity with a risk of acute kidney injury (AKI). Real-world data on the safety of NSAID use in CKD stages 4–5 in the context of endoscopic procedures is limited. We aimed to assess and weigh the renal safety with the incidence of AKI following NSAIDs use vs the prophylactic benefit of protecting against PEP in advanced CKD patients.
Methods: Utilizing the TriNetX US Collaborative Network, we identified CKD stage 4 and 5 patients (with GFR < 30, excluding those on dialysis/renal replacement therapy) who underwent ERCP and received rectal NSAIDs prophylaxis during the procedure, and compared them to another matched group of CKD patients who underwent ERCP and did not receive prophylaxis. The primary outcome was the development of AKI following the ERCP. Secondary outcomes included PEP, gastrointestinal bleeding (GIB), ICU admission, acute necrotizing pancreatitis (ANP), sepsis, intubation, and mortality. We matched the groups based on baseline characteristics, co-morbidities, and pertinent medications that increase the risk of kidney injury (including diuretics, ACEi, ARBs, etc) to rule out potential confounders.
Results: A total of 1,490 patients with CKD stages 4 or 5 received NSAIDs and 3,773 patients who did not were identified before propensity score matching, all of whom underwent ERCP. After matching, each group included 1,470 patients with comparable baseline characteristics. (Table 1).
There was no significant difference in the incidence of AKI between both groups (13% vs. 12%, p = 0.243). However, PEP was less common in the NSAID group (8% vs. 10%, p = 0.027). There was no difference in rates of GIB, intubation, sepsis, or mortality. Notably, ANP occurred only in the non-NSAIDs group (0% vs. 0.7%, p = 0.002). (Table 2)
Discussion: In patients with advanced CKD (GFR< 30) undergoing ERCP, NSAIDs were not linked to higher risks of AKI, GI bleeding, or mortality; they were associated with lower PEP risk. These findings suggest that, with appropriate patient selection, NSAIDs may be safely used in this high risk patient population for single doses.

Figure: Table 1: baseline characteristics, co-morbidities, and pertinent medications before and after propensity score matching

Figure: Table 2: outcomes
Disclosures:
Mohammed Abusuliman indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Amr Abusuliman indicated no relevant financial relationships.
Mohamed Eldesouki indicated no relevant financial relationships.
Mohammed Y. Youssef indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Jonathan Montrose indicated no relevant financial relationships.
Anas Elsaka indicated no relevant financial relationships.
Mona Ali indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Mark Gromski: Grant/Research/Clinical Trial Support – Allurion; Cook Medical; Fractyl. Consultant/Advisory Boards – Ambu; Boston Scientific; Cook Medical – Advisory Committee/Board Member, Grant/Research Support.
Sherif Elhanafi indicated no relevant financial relationships.
Mohammed Abusuliman, MD1, Azizullah Beran, MD2, Khaled Elfert, MBChB, MRCP3, Amr Abusuliman, MD4, Mohamed Eldesouki, MD5, Mohammed Y. Youssef, MD6, Hazem Abosheaishaa, MD7, Jonathan A. Montrose, DO8, Anas A. Elsaka, MD9, Mona A. Ali, MD9, Ahmed Salem, MD10, Mark Gromski, MD2, Sherif E. Elhanafi, MD11. P1408 - The Safety of Peri-ERCP Rectal Prophylactic Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Patients With Advanced Chronic Kidney Disease (GFR < 30), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
