Sunday Poster Session
Category: Interventional Endoscopy
P1404 - Endoscopic Gastropexy in High-Risk Patients: A Single Center Experience
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Hadi Khaled Abou Zeid, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Alwatheq Al-Itelat, MD1, Moataz Aboeldahb, MBBCh2, Khusboo Gala, MD1, Andrew Storm, MD1
1Mayo Clinic, Rochester, MN; 2Mayo Clinic College of Medicine and Science, Rochester, MN
Introduction: Endoscopic gastropexy is performed to anchor the gastric wall to the abdominal wall and subsequently prevent recurrence of gastric volvulus or manage paraesophageal hernias. While laparoscopic approaches are well established, endoscopic gastropexy has gained attention as a less invasive alternative, particularly in high-risk or elderly patients, who are not ideal surgical candidates. However, data on its safety, clinical efficacy, and long-term outcomes remain limited. In this study, we aim to evaluate the safety and efficacy of endoscopic gastropexy in a cohort of high-risk patients treated at our institution.
Methods: We conducted a retrospective review of patients who underwent endoscopic gastropexy at our center between 2020 and 2024. Data were collected on patient demographics, comorbidities, procedural details, and outcomes. Endoscopic gastropexy was performed using one or two PEG tubes to anchor the anterior wall of the stomach to the abdominal wall under endoscopic and transillumination guidance. Technical, clinical, and long-term success, in addition to complications were assessed.
Results: A total of 9 patients (mean age 77 ±14.9, 12.5% male) underwent endoscopic gastropexy. Technical and clinical success rates were achieved in 100 and 75%, respectively. No intraprocedural complications were observed. Postprocedural complications occurred in 44.4% (4/9) of patients and included hematoma formation (2/4), volvulus (1/4), and aspiration pneumonitis (1/4). Long-term success was achieved in 62.5% of patients, while mortality within 30 days post-procedure was observed in one patient (12.5%).
Discussion: Endoscopic gastropexy is a feasible and effective option for the management of high-risk patients. Nonetheless, the notable rate of postprocedural complications highlights the need for further prospective studies to optimize patient selection and procedural outcomes.
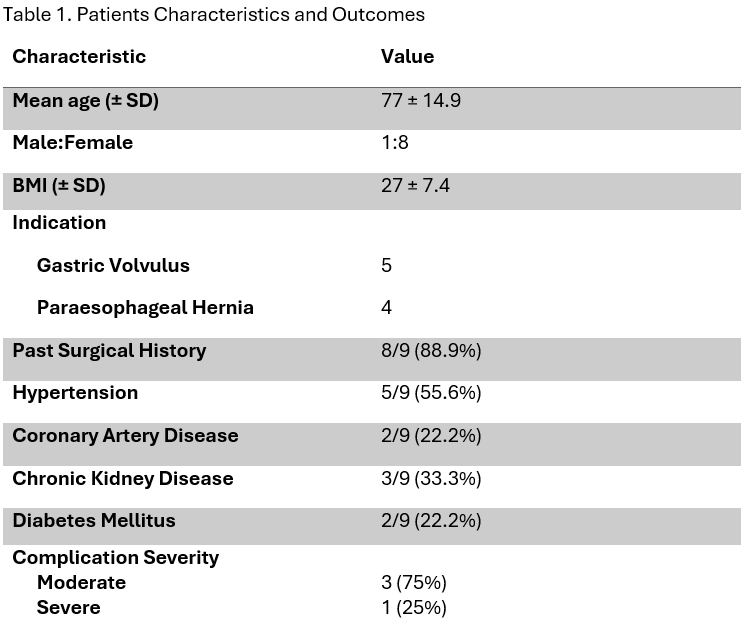
Figure: Table 1. Patients Characteristics and Outcomes
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Alwatheq Al-Itelat indicated no relevant financial relationships.
Moataz Aboeldahb indicated no relevant financial relationships.
Khusboo Gala indicated no relevant financial relationships.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Alwatheq Al-Itelat, MD1, Moataz Aboeldahb, MBBCh2, Khusboo Gala, MD1, Andrew Storm, MD1. P1404 - Endoscopic Gastropexy in High-Risk Patients: A Single Center Experience, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Mayo Clinic College of Medicine and Science, Rochester, MN
Introduction: Endoscopic gastropexy is performed to anchor the gastric wall to the abdominal wall and subsequently prevent recurrence of gastric volvulus or manage paraesophageal hernias. While laparoscopic approaches are well established, endoscopic gastropexy has gained attention as a less invasive alternative, particularly in high-risk or elderly patients, who are not ideal surgical candidates. However, data on its safety, clinical efficacy, and long-term outcomes remain limited. In this study, we aim to evaluate the safety and efficacy of endoscopic gastropexy in a cohort of high-risk patients treated at our institution.
Methods: We conducted a retrospective review of patients who underwent endoscopic gastropexy at our center between 2020 and 2024. Data were collected on patient demographics, comorbidities, procedural details, and outcomes. Endoscopic gastropexy was performed using one or two PEG tubes to anchor the anterior wall of the stomach to the abdominal wall under endoscopic and transillumination guidance. Technical, clinical, and long-term success, in addition to complications were assessed.
Results: A total of 9 patients (mean age 77 ±14.9, 12.5% male) underwent endoscopic gastropexy. Technical and clinical success rates were achieved in 100 and 75%, respectively. No intraprocedural complications were observed. Postprocedural complications occurred in 44.4% (4/9) of patients and included hematoma formation (2/4), volvulus (1/4), and aspiration pneumonitis (1/4). Long-term success was achieved in 62.5% of patients, while mortality within 30 days post-procedure was observed in one patient (12.5%).
Discussion: Endoscopic gastropexy is a feasible and effective option for the management of high-risk patients. Nonetheless, the notable rate of postprocedural complications highlights the need for further prospective studies to optimize patient selection and procedural outcomes.

Figure: Table 1. Patients Characteristics and Outcomes
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Alwatheq Al-Itelat indicated no relevant financial relationships.
Moataz Aboeldahb indicated no relevant financial relationships.
Khusboo Gala indicated no relevant financial relationships.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Alwatheq Al-Itelat, MD1, Moataz Aboeldahb, MBBCh2, Khusboo Gala, MD1, Andrew Storm, MD1. P1404 - Endoscopic Gastropexy in High-Risk Patients: A Single Center Experience, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
