Sunday Poster Session
Category: Interventional Endoscopy
P1368 - Cost-Effectiveness Analysis of Endoscopic Ultrasound-Guided Liver Biopsy Compared to Percutaneous Liver Biopsy in Patients With and Without Cirrhosis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MH
Muhammad Haseeb, MD
University of Pittsburgh Medical Center
Pittsburgh, PA
Presenting Author(s)
Muhammad Haseeb, MD1, Maham Waqar, MD2, Hafsah Ijaz, MD3, Muhammad Kamal, MD4, Fnu Faheela, MBBS5, Christopher Thompson, MD6, Pichamol Jirapinyo, MD7
1University of Pittsburgh Medical Center, Pittsburgh, PA; 2Allegheny General Hospital, Pittsburgh, PA; 3Capital Health New Jersey, Trenton, NJ; 4Hackensack Meridian Health, Edison, NJ; 5Nishtar Medical University, Multan, Punjab, Pakistan; 6Brigham and Women's Hospital, Boston, MA; 7Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Introduction: Percutaneous liver biopsy (PC-LB) is an established, minimally invasive technique for obtaining liver tissue samples for the diagnosis and risk stratification of liver disease. More recently, endoscopic ultrasound-guided liver biopsy (EUS-LB) has emerged as a comparable alternative to PC-LB, offering similar safety and efficacy profiles with potential advantages, including superior tissue acquisition, reduced recovery time, and less post-procedural pain. Despite these benefits, limited data are available on the cost-effectiveness of EUS-LB relative to PC-LB. Therefore, we aimed to evaluate the cost-effectiveness of EUS-LB compared to PC-LB in patients with and without advanced fibrosis or cirrhosis.
Methods: A state-transition Markov cohort model was constructed to evaluate the cost-effectiveness of EUS-LB compared to PC-LB from the U.S. healthcare system’s perspective. The base case was a 55-year-old patient undergoing liver biopsy for any clinical indication without known cirrhosis. Model inputs, including probabilities, costs (US $ 2024), and quality of life (QOL) estimates, were derived from published literature. Health outcomes expressed in quality-adjusted life years (QALYs), model was run over one-year time horizon with a cycle length of one month and a 3% discount rate was utilized. The primary outcome measure was the incremental cost-effectiveness ratio (ICER) with a willingness-to-pay threshold (WTP) of $100,000/QALY. One-way and probabilistic sensitivity analyses were conducted.
Results: At 1 year, EUS-LB demonstrated greater effectiveness than PC-LB (0.86 QALY vs. 0.85 QALY), with an additional cost of $658 per patient. EUS-LB was determined to be cost-effective compared to PC-LB, with an ICER of $58,770/QALY and a WTP threshold of $100,000/QALY. One-way sensitivity analysis confirmed the robustness of these findings, while probabilistic sensitivity analysis, using 10,000 iterations, showed EUS-LB to be cost-effective with a 77% probability. Additionally, in scenario analysis, EUS-LB was cost-saving for patients with advanced fibrosis or cirrhosis compared to PC-LB.
Discussion: EUS-LB is a cost-effective alternative to PC-LB for patients requiring liver biopsy and is cost-saving in those with advanced fibrosis or cirrhosis, as it eliminates the need for subsequent endoscopic variceal screening. These findings underscore the potential of EUS-LB as a multi-purpose, cost-effective modality that reduces the need for multiple procedures while improving patient quality of life.
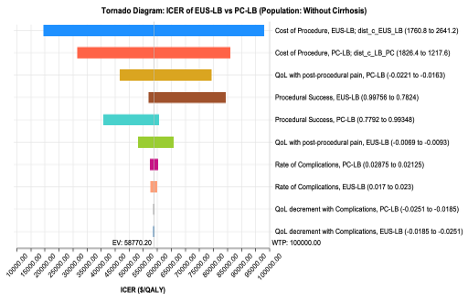
Figure: Table 1: a) Base Case Model Inputs b) Base Case Results
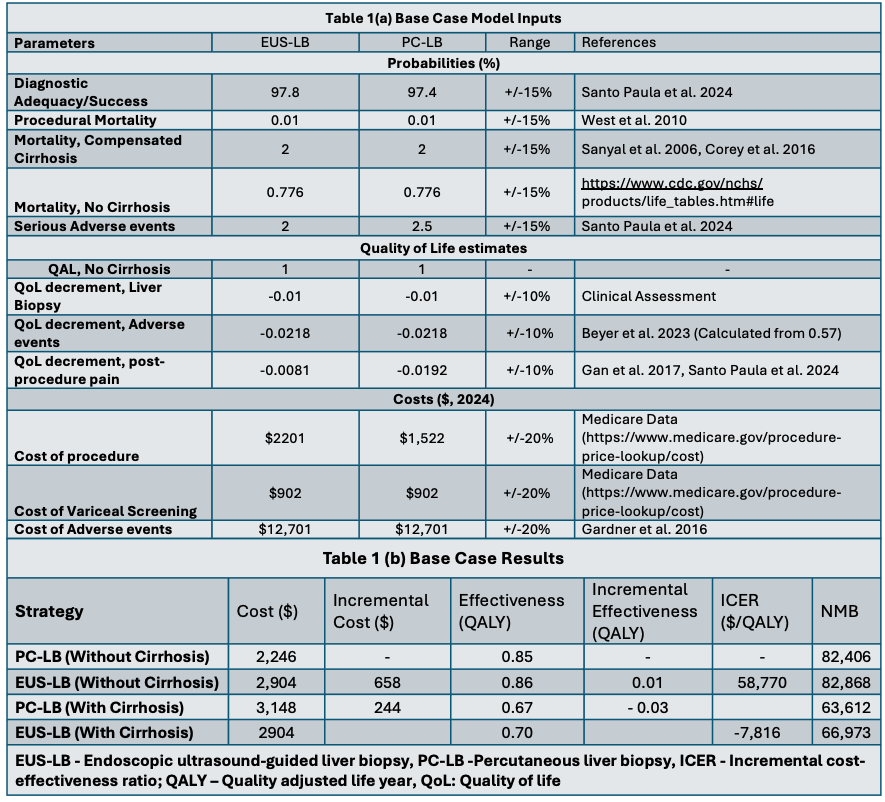
Figure: Tornado Diagram: One-way Sensitivity Analysis of ICER of EUS-LB vs. PC-LB with key input parameters.
Disclosures:
Muhammad Haseeb indicated no relevant financial relationships.
Maham Waqar indicated no relevant financial relationships.
Hafsah Ijaz indicated no relevant financial relationships.
Muhammad Kamal indicated no relevant financial relationships.
Fnu Faheela indicated no relevant financial relationships.
Christopher Thompson indicated no relevant financial relationships.
Pichamol Jirapinyo indicated no relevant financial relationships.
Muhammad Haseeb, MD1, Maham Waqar, MD2, Hafsah Ijaz, MD3, Muhammad Kamal, MD4, Fnu Faheela, MBBS5, Christopher Thompson, MD6, Pichamol Jirapinyo, MD7. P1368 - Cost-Effectiveness Analysis of Endoscopic Ultrasound-Guided Liver Biopsy Compared to Percutaneous Liver Biopsy in Patients With and Without Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Pittsburgh Medical Center, Pittsburgh, PA; 2Allegheny General Hospital, Pittsburgh, PA; 3Capital Health New Jersey, Trenton, NJ; 4Hackensack Meridian Health, Edison, NJ; 5Nishtar Medical University, Multan, Punjab, Pakistan; 6Brigham and Women's Hospital, Boston, MA; 7Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Introduction: Percutaneous liver biopsy (PC-LB) is an established, minimally invasive technique for obtaining liver tissue samples for the diagnosis and risk stratification of liver disease. More recently, endoscopic ultrasound-guided liver biopsy (EUS-LB) has emerged as a comparable alternative to PC-LB, offering similar safety and efficacy profiles with potential advantages, including superior tissue acquisition, reduced recovery time, and less post-procedural pain. Despite these benefits, limited data are available on the cost-effectiveness of EUS-LB relative to PC-LB. Therefore, we aimed to evaluate the cost-effectiveness of EUS-LB compared to PC-LB in patients with and without advanced fibrosis or cirrhosis.
Methods: A state-transition Markov cohort model was constructed to evaluate the cost-effectiveness of EUS-LB compared to PC-LB from the U.S. healthcare system’s perspective. The base case was a 55-year-old patient undergoing liver biopsy for any clinical indication without known cirrhosis. Model inputs, including probabilities, costs (US $ 2024), and quality of life (QOL) estimates, were derived from published literature. Health outcomes expressed in quality-adjusted life years (QALYs), model was run over one-year time horizon with a cycle length of one month and a 3% discount rate was utilized. The primary outcome measure was the incremental cost-effectiveness ratio (ICER) with a willingness-to-pay threshold (WTP) of $100,000/QALY. One-way and probabilistic sensitivity analyses were conducted.
Results: At 1 year, EUS-LB demonstrated greater effectiveness than PC-LB (0.86 QALY vs. 0.85 QALY), with an additional cost of $658 per patient. EUS-LB was determined to be cost-effective compared to PC-LB, with an ICER of $58,770/QALY and a WTP threshold of $100,000/QALY. One-way sensitivity analysis confirmed the robustness of these findings, while probabilistic sensitivity analysis, using 10,000 iterations, showed EUS-LB to be cost-effective with a 77% probability. Additionally, in scenario analysis, EUS-LB was cost-saving for patients with advanced fibrosis or cirrhosis compared to PC-LB.
Discussion: EUS-LB is a cost-effective alternative to PC-LB for patients requiring liver biopsy and is cost-saving in those with advanced fibrosis or cirrhosis, as it eliminates the need for subsequent endoscopic variceal screening. These findings underscore the potential of EUS-LB as a multi-purpose, cost-effective modality that reduces the need for multiple procedures while improving patient quality of life.

Figure: Table 1: a) Base Case Model Inputs b) Base Case Results

Figure: Tornado Diagram: One-way Sensitivity Analysis of ICER of EUS-LB vs. PC-LB with key input parameters.
Disclosures:
Muhammad Haseeb indicated no relevant financial relationships.
Maham Waqar indicated no relevant financial relationships.
Hafsah Ijaz indicated no relevant financial relationships.
Muhammad Kamal indicated no relevant financial relationships.
Fnu Faheela indicated no relevant financial relationships.
Christopher Thompson indicated no relevant financial relationships.
Pichamol Jirapinyo indicated no relevant financial relationships.
Muhammad Haseeb, MD1, Maham Waqar, MD2, Hafsah Ijaz, MD3, Muhammad Kamal, MD4, Fnu Faheela, MBBS5, Christopher Thompson, MD6, Pichamol Jirapinyo, MD7. P1368 - Cost-Effectiveness Analysis of Endoscopic Ultrasound-Guided Liver Biopsy Compared to Percutaneous Liver Biopsy in Patients With and Without Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
