Sunday Poster Session
Category: Infections and Microbiome
P1297 - Efficacy of Fecal Microbiota Transplant for Prevention of Recurrent CDI in Patients Diagnosed With Cancer
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Maria Julia M. N Santos, MD (she/her/hers)
MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Maria Julia M. N.. Santos, MD1, Kristin Junek, ARNP1, Sharada Wali, MBBS, MPH2, Reema Patel, MD3, Carolina Cruz, MD2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas Medical Branch, Galveston, TX
Introduction: The gut microbiota is essential for gastrointestinal function and immune regulation. Disruption of this microbial environment increases susceptibility to gastrointestinal infections, including Clostridioides difficile infection (CDI). Fecal microbiota transplantation (FMT) restores microbial diversity and is approved for preventing CDI in high-risk individuals with multiple recurrences. Delivery methods include colonoscopy, oral FMT spore capsules (VOWST),and rectal enema (Rebyota). Studies evaluating the efficacy of FMT among cancer population with frequent immunosuppression and antibiotic treatment remain limited.
Methods: This is a retrospective study among patients living with cancer at our single center who received FMT between October 2017 and March 2025. Clinical and cancer-related data were collected at baseline, along with information related to CDI.
Results: A total of 69 patients with a history of CDI or rCDI were included. Hematologic malignancies were the most common cancer type (29.6%), and nearly half had stage III–IV disease. At the time of their most recent CDI episode, 55% were receiving active cancer therapy. Vancomycin and fidaxomicin were the most frequently used antibiotics, and 18.8% received bezlotoxumab.
Colonoscopy-delivered FMT was the most common intervention (68.1%), followed by Rebyota (17.4%) and VOWST (7.2%); combination strategies were used in 7.2%. 90-day recurrence was observed in 4 patients (5.8%), and recurrence at any time during follow-up of 11 months occurred in 13 patients (18.8%). In a subgroup analysis of 59 patients treated with either colonoscopy-delivered FMT or Rebyota, baseline characteristics were similar, and 90-day recurrence was low and comparable (6.4% vs. 8.3%). Overall, adverse events were rare (2.9%) and limited to mild and self-limiting symptoms. The median time to recurrence was 3 months. Cancer progression was reported in 36.2% patients at the time of FMT, which dropped to 26.1% at the last follow up. The all-cause mortality was 40.6%. Univariate analysis did not identify statistically significant predictors of rCDI.
Discussion: In this retrospective study of fecal microbiota-based therapy for CDI among high-risk populations, recurrence within 90 days was low across all delivery methods at 5.8%. These findings support the high efficacy and favorable safety of microbiota-based therapies for rCDI prevention in oncology population.
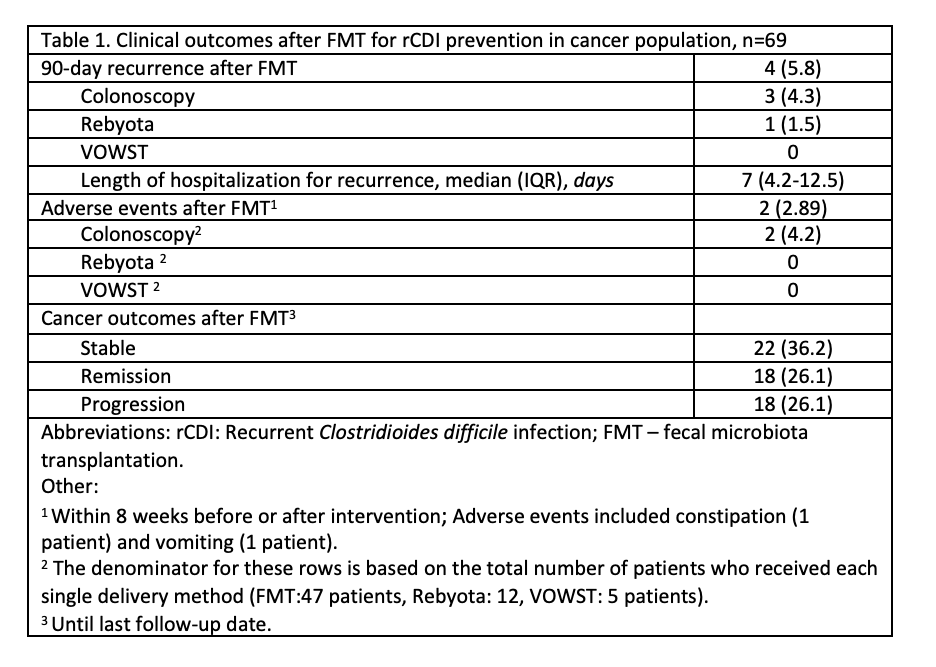
Figure: Table 1. Clinical outcomes after FMT for rCDI prevention in cancer population, n=69
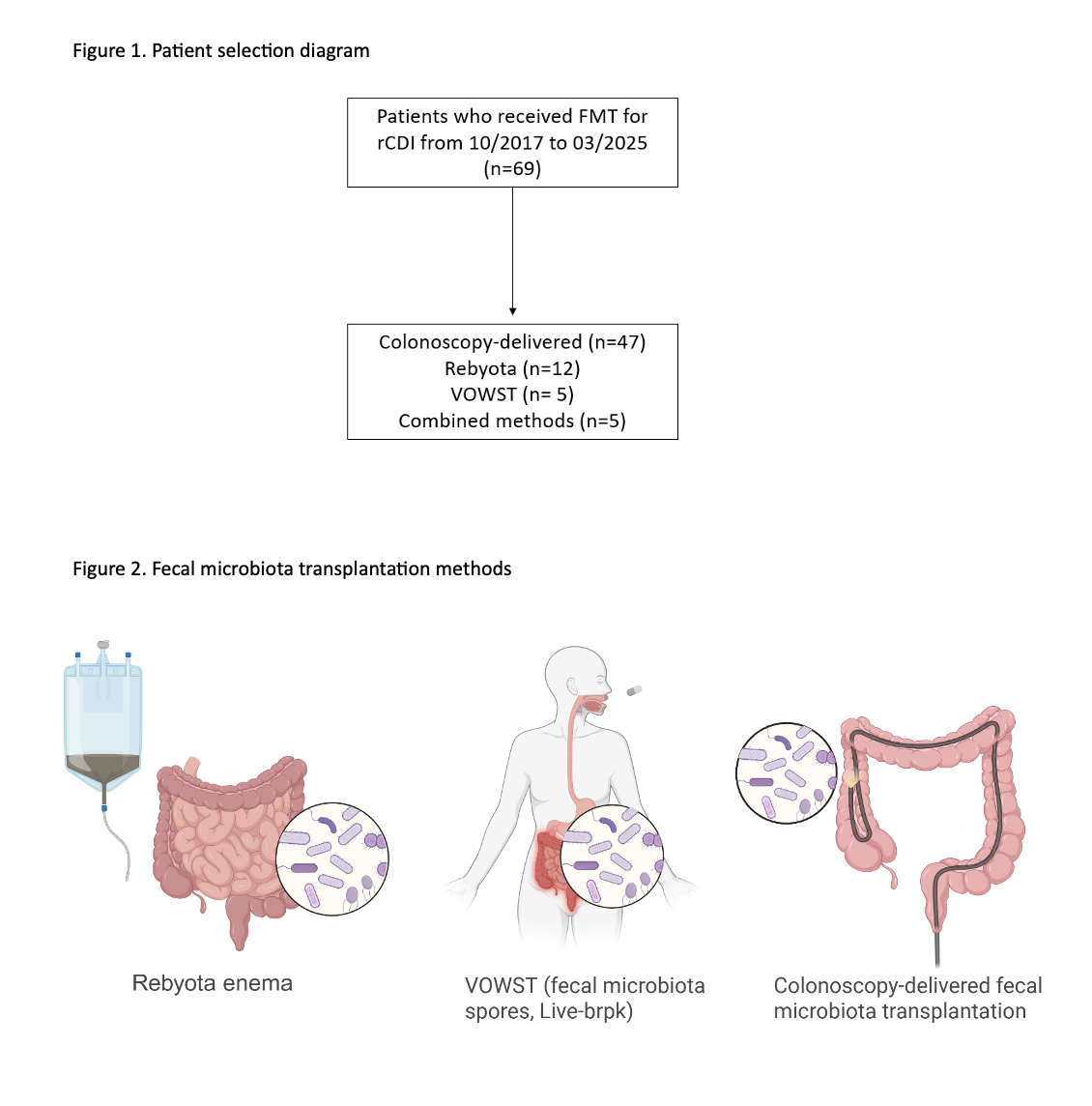
Figure: Patient selection and methods of FMT
Disclosures:
Maria Julia Santos indicated no relevant financial relationships.
Kristin Junek indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Reema Patel indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Maria Julia M. N.. Santos, MD1, Kristin Junek, ARNP1, Sharada Wali, MBBS, MPH2, Reema Patel, MD3, Carolina Cruz, MD2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2. P1297 - Efficacy of Fecal Microbiota Transplant for Prevention of Recurrent CDI in Patients Diagnosed With Cancer, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas Medical Branch, Galveston, TX
Introduction: The gut microbiota is essential for gastrointestinal function and immune regulation. Disruption of this microbial environment increases susceptibility to gastrointestinal infections, including Clostridioides difficile infection (CDI). Fecal microbiota transplantation (FMT) restores microbial diversity and is approved for preventing CDI in high-risk individuals with multiple recurrences. Delivery methods include colonoscopy, oral FMT spore capsules (VOWST),and rectal enema (Rebyota). Studies evaluating the efficacy of FMT among cancer population with frequent immunosuppression and antibiotic treatment remain limited.
Methods: This is a retrospective study among patients living with cancer at our single center who received FMT between October 2017 and March 2025. Clinical and cancer-related data were collected at baseline, along with information related to CDI.
Results: A total of 69 patients with a history of CDI or rCDI were included. Hematologic malignancies were the most common cancer type (29.6%), and nearly half had stage III–IV disease. At the time of their most recent CDI episode, 55% were receiving active cancer therapy. Vancomycin and fidaxomicin were the most frequently used antibiotics, and 18.8% received bezlotoxumab.
Colonoscopy-delivered FMT was the most common intervention (68.1%), followed by Rebyota (17.4%) and VOWST (7.2%); combination strategies were used in 7.2%. 90-day recurrence was observed in 4 patients (5.8%), and recurrence at any time during follow-up of 11 months occurred in 13 patients (18.8%). In a subgroup analysis of 59 patients treated with either colonoscopy-delivered FMT or Rebyota, baseline characteristics were similar, and 90-day recurrence was low and comparable (6.4% vs. 8.3%). Overall, adverse events were rare (2.9%) and limited to mild and self-limiting symptoms. The median time to recurrence was 3 months. Cancer progression was reported in 36.2% patients at the time of FMT, which dropped to 26.1% at the last follow up. The all-cause mortality was 40.6%. Univariate analysis did not identify statistically significant predictors of rCDI.
Discussion: In this retrospective study of fecal microbiota-based therapy for CDI among high-risk populations, recurrence within 90 days was low across all delivery methods at 5.8%. These findings support the high efficacy and favorable safety of microbiota-based therapies for rCDI prevention in oncology population.

Figure: Table 1. Clinical outcomes after FMT for rCDI prevention in cancer population, n=69

Figure: Patient selection and methods of FMT
Disclosures:
Maria Julia Santos indicated no relevant financial relationships.
Kristin Junek indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Reema Patel indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Maria Julia M. N.. Santos, MD1, Kristin Junek, ARNP1, Sharada Wali, MBBS, MPH2, Reema Patel, MD3, Carolina Cruz, MD2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2. P1297 - Efficacy of Fecal Microbiota Transplant for Prevention of Recurrent CDI in Patients Diagnosed With Cancer, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
