Sunday Poster Session
Category: IBD
P1210 - Subcutaneous Mirikizumab Maintenance Treatment Over 40 Weeks Leads to Incremental Improvements in Rates of Clinical Remission and Endoscopic Response: Results From the Phase 3 VIVID-1 Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- SM
Sebastian Maier
Lilly Deutschland GmbH
Bad Homburg, Hessen, Germany
Presenting Author(s)
Peter Bossuyt, 1, David Laharie, 2, Stephan Brand, 3, Dominik Bettenworth, MD4, James Lindsey, PhD, BM, BCh5, Sebastian Maier, 6, Marijana Protic, 7, Emily Hon, 7, Hilde Carlier, 8, Huaiyu Zang, 9, Guanglei Yu, PhD10, Walter Reinisch, MD, PhD11
1Imelda General Hospital, Bonheiden, Antwerpen, Belgium; 2CHU de Bordeaux, Centre Medico-chirurgical Magellan, Hôpital Haut-Lévêque, Gastroenterology Department; Université de Bordeaux, Pessac, Aquitaine, France; 3Department of Gastroenterology and Hepatology, HOCH, Cantonal Hospital St. Gallen, St. Gallen, Sankt Gallen, Switzerland; 4IBD Focus Practice of Excellence, Münster, Münster, Nordrhein-Westfalen, Germany; 5Digestive Disorders Clinical Academic Unit, Barta & The London School of Medicine, Department of Digestive Diseases, London, England, United Kingdom; 6Lilly Deutschland GmbH, Bad Homburg, Hessen, Germany; 7Eli Lilly and Company, Indianapolis, IN; 8Eli Lilly and Company, Markiesstraat, Brussels Hoofdstedelijk Gewest, Belgium; 9Tigermed-BDM Inc., Somerset, NJ; 10Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 11Medical University of Vienna, Department of Internal Medicine III, Division of Gastroenterology and Hepatology, Spitalgasse, Wien, Austria
Introduction: Mirikizumab, a p19-directed interleukin-23 monoclonal antibody, is efficacious in inducing clinical response at Week(W) 12 and maintaining clinical remission through W52 in moderately-to-severely active Crohn’s disease (CD) as shown in the phase 3 treat-through trial VIVID-1 (NCT03926130). Here, we present analysis of maintenance outcomes through W52 for Patient-Reported Outcome (PRO) clinical responders and nonresponders at W12.
Methods: The analysis set includes patients randomized to mirikizumab at baseline, who did or did not achieve PRO clinical response at W12 after intravenous (IV) induction treatment (900mg mirikizumab IV every 4 weeks [Q4W]) before entering subcutaneous (SC) maintenance treatment (300mg mirikizumab SC Q4W). Descriptive statistics are used to summarize the data, applying nonresponder imputation for missing binary endpoints. As a treat-through study, no rerandomization was applied.
Results: From a total of 551 patients who entered SC maintenance treatment, baseline characteristics of age, duration of CD, and baseline Crohn’s Disease Activity Index (CDAI) were similar between W12 PRO responders (n=402, 73.0%) and W12 PRO nonresponders (n=149, 27.0%). PRO responders at W12 had a higher objective inflammatory burden at baseline (faecal calprotectin, c-reactive protein, and Simple Endoscopic Score-CD), whereas concomitant or prior medication usage was similar between the groups. For W12 PRO responders, SC mirikizumab treatment from W12 to W52 resulted in an increase in CDAI clinical remission from 52.2% to 65.4%, PRO clinical remission from 53.2% to 65.4%, and endoscopic response from 35.1% to 54.7%, respectively (Table). Among W12 PRO nonresponders, PRO response was achieved by 39.6% at W16, 45.6% at W20, 44.3% at W24, and 56.4% at W52 with continued mirikizumab SC treatment, while CDAI clinical remission and PRO clinical remission were achieved by 33.6% and 31.5% at W52, respectively. Endoscopic response increased from 30.2% at W12 to 40.3% at W52 among W12 PRO nonresponders (Table).
Discussion: Subcutaneous mirikizumab maintenance treatment over 40 weeks leads to incremental and sustained improvements in rates of clinical and endoscopic response regardless of PRO clinical response status after induction. A substantial proportion of clinical nonresponders at W12 improved early during maintenance treatment suggesting continued treatment with mirikizumab can be beneficial and lead to long-term response.
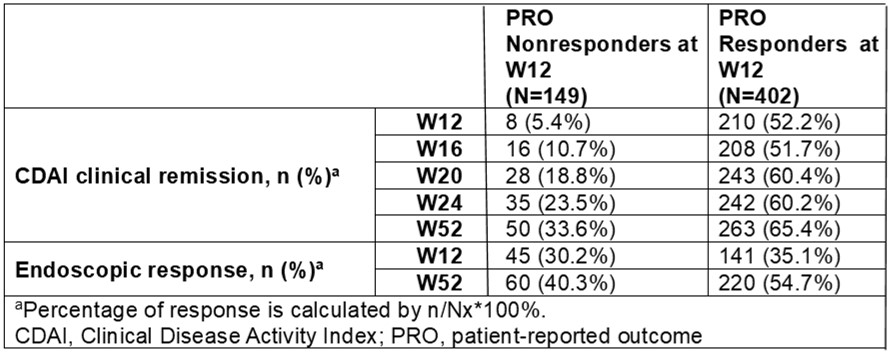
Figure: CDAI Clinical Remission and Endoscopic Response by W12 PRO Response Status
Disclosures:
Peter Bossuyt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Amgen – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Lecture fees. CAG – Lecture fees. Celltrion – Advisory Committee/Board Member, Lecture fees. CIRC – Advisory Committee/Board Member. Dr. Falk Pharma Benelux – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Lecture fees. EPGS – Lecture fees. Galapagos NV – Advisory Committee/Board Member, Lecture fees. Globalport – Lecture fees. Janssen – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Janssen – Lecture fees. Materia Prima – Lecture fees. Pentax – Advisory Committee/Board Member, Lecture fees. Pfizer – Grant/Research Support. PSI-CRO – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Scope – Lecture fees. Takeda – Advisory Committee/Board Member, Lecture fees. Tetrameros – Advisory Committee/Board Member. Viatris – Grant/Research Support.
David Laharie: AbbVie – Advisory Committee/Board Member, Consultant, Transport, Fees.. Alfasigma – Advisory Committee/Board Member, Consultant, Transport or Fees. Amgen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Celltrion – Advisory Committee/Board Member, Consultant, Transport, Fees.. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Transport, Fees.. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Transport, Fees.. Janssen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Medac – Advisory Committee/Board Member, Consultant, Transport or Fees. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Transport, Fees.. Pfizer – Advisory Committee/Board Member, Consultant, Transport, Fees.. Prometheus – Advisory Committee/Board Member, Consultant, Transport, Fees.. Takeda – Advisory Committee/Board Member, Consultant, Transport, Fees.. Theradiag – Advisory Committee/Board Member, Consultant, Transport, Fees..
Stephan Brand: Abbvie – Advisory Committee/Board Member, Speakers Bureau. BMS – Advisory Committee/Board Member. Celgene – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Falk Foundation – Speakers Bureau. Ferring – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. MSD – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Pierre Fabre – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Educational grant, Speakers Bureau. UCB – Advisory Committee/Board Member, Speakers Bureau. Vifor – Speakers Bureau.
Dominik Bettenworth: AbbVie – Advisory Committee/Board Member, Consultant. Alfasigma – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Arena – Advisory Committee/Board Member, Consultant. Art tempi – Advisory Committee/Board Member, Consultant. BNG Service GmbH – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. CED-Service GmbH – Advisory Committee/Board Member, Consultant. Celltrion – Advisory Committee/Board Member, Consultant. DGVS – Advisory Committee/Board Member, Consultant. Diaplan – Advisory Committee/Board Member, Consultant. Doctorflix – Advisory Committee/Board Member, Consultant. Else Kröner-Fresenius Foundation – Advisory Committee/Board Member, Consultant. Falk Foundation – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Fresenius – Advisory Committee/Board Member, Consultant. Galapagos – Advisory Committee/Board Member, Consultant. Gastro Today – Advisory Committee/Board Member, Consultant. GSK – Advisory Committee/Board Member, Consultant. Guidepoint – Advisory Committee/Board Member, Consultant. Hexal – Advisory Committee/Board Member, Consultant. Impulze – Advisory Committee/Board Member, Consultant. Janssen Cilag – Advisory Committee/Board Member, Consultant. Lilly – Advisory Committee/Board Member, Consultant. Medical Tribune – Advisory Committee/Board Member, Consultant. MedTriX – Advisory Committee/Board Member, Consultant. MSD – Advisory Committee/Board Member, Consultant. Mylan – Advisory Committee/Board Member, Consultant. Onkowissen – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Stada – Advisory Committee/Board Member, Consultant. StreamedUp – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Tetrameros – Advisory Committee/Board Member, Consultant. Thieme – Advisory Committee/Board Member, Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Biopiharma – Advisory Committee/Board Member, Consultant. Viatris – Advisory Committee/Board Member, Consultant. Vifor – Advisory Committee/Board Member, Consultant.
James Lindsey: AbbVie – Consultant, Grant/Research Support, Educational grant, Speakers Bureau. Amgen – Educational grant. Astra Zeneca – Consultant. BMS – Consultant, Educational grant. Celgene – Consultant. Celtrion – Consultant. Eli Lilly and Company – Consultant, Speakers Bureau. Engytix – Consultant. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Educational grant, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Educational grant. GSK – Consultant. Honoraria for developing and delivering the Cornerstones Health Best of DDW program – Educational grants. Janssen – Consultant, Educational grant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Educational grant, Speakers Bureau. Sandoz – Educational grant. Takeda – Consultant, Educational grant, Speakers Bureau. Teva – Consultant. Tillot’s – Educational grant, Speakers Bureau.
Sebastian Maier: Eli Lilly and Company – Employee, Stock Options.
Marijana Protic: Eli Lilly and Company – Employee, Stock Options.
Emily Hon: Eli Lilly and Company – Employee, Stock Options.
Hilde Carlier: Eli Lilly and Company – Employee, Stock Options.
Huaiyu Zang: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Walter Reinisch: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Actelion – Advisory Committee/Board Member, Consultant. Alpha Wasserman – Advisory Committee/Board Member, Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Cellerix – Advisory Committee/Board Member, Consultant. Cosmo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Grunenthal – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant. Millennium – Advisory Committee/Board Member, Consultant. Novo Nordisk – Advisory Committee/Board Member, Consultant. Nycomed – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Schering-Plough – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB Pharma – Advisory Committee/Board Member, Consultant. Vifor Pharma – Advisory Committee/Board Member, Consultant.
Peter Bossuyt, 1, David Laharie, 2, Stephan Brand, 3, Dominik Bettenworth, MD4, James Lindsey, PhD, BM, BCh5, Sebastian Maier, 6, Marijana Protic, 7, Emily Hon, 7, Hilde Carlier, 8, Huaiyu Zang, 9, Guanglei Yu, PhD10, Walter Reinisch, MD, PhD11. P1210 - Subcutaneous Mirikizumab Maintenance Treatment Over 40 Weeks Leads to Incremental Improvements in Rates of Clinical Remission and Endoscopic Response: Results From the Phase 3 VIVID-1 Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Imelda General Hospital, Bonheiden, Antwerpen, Belgium; 2CHU de Bordeaux, Centre Medico-chirurgical Magellan, Hôpital Haut-Lévêque, Gastroenterology Department; Université de Bordeaux, Pessac, Aquitaine, France; 3Department of Gastroenterology and Hepatology, HOCH, Cantonal Hospital St. Gallen, St. Gallen, Sankt Gallen, Switzerland; 4IBD Focus Practice of Excellence, Münster, Münster, Nordrhein-Westfalen, Germany; 5Digestive Disorders Clinical Academic Unit, Barta & The London School of Medicine, Department of Digestive Diseases, London, England, United Kingdom; 6Lilly Deutschland GmbH, Bad Homburg, Hessen, Germany; 7Eli Lilly and Company, Indianapolis, IN; 8Eli Lilly and Company, Markiesstraat, Brussels Hoofdstedelijk Gewest, Belgium; 9Tigermed-BDM Inc., Somerset, NJ; 10Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 11Medical University of Vienna, Department of Internal Medicine III, Division of Gastroenterology and Hepatology, Spitalgasse, Wien, Austria
Introduction: Mirikizumab, a p19-directed interleukin-23 monoclonal antibody, is efficacious in inducing clinical response at Week(W) 12 and maintaining clinical remission through W52 in moderately-to-severely active Crohn’s disease (CD) as shown in the phase 3 treat-through trial VIVID-1 (NCT03926130). Here, we present analysis of maintenance outcomes through W52 for Patient-Reported Outcome (PRO) clinical responders and nonresponders at W12.
Methods: The analysis set includes patients randomized to mirikizumab at baseline, who did or did not achieve PRO clinical response at W12 after intravenous (IV) induction treatment (900mg mirikizumab IV every 4 weeks [Q4W]) before entering subcutaneous (SC) maintenance treatment (300mg mirikizumab SC Q4W). Descriptive statistics are used to summarize the data, applying nonresponder imputation for missing binary endpoints. As a treat-through study, no rerandomization was applied.
Results: From a total of 551 patients who entered SC maintenance treatment, baseline characteristics of age, duration of CD, and baseline Crohn’s Disease Activity Index (CDAI) were similar between W12 PRO responders (n=402, 73.0%) and W12 PRO nonresponders (n=149, 27.0%). PRO responders at W12 had a higher objective inflammatory burden at baseline (faecal calprotectin, c-reactive protein, and Simple Endoscopic Score-CD), whereas concomitant or prior medication usage was similar between the groups. For W12 PRO responders, SC mirikizumab treatment from W12 to W52 resulted in an increase in CDAI clinical remission from 52.2% to 65.4%, PRO clinical remission from 53.2% to 65.4%, and endoscopic response from 35.1% to 54.7%, respectively (Table). Among W12 PRO nonresponders, PRO response was achieved by 39.6% at W16, 45.6% at W20, 44.3% at W24, and 56.4% at W52 with continued mirikizumab SC treatment, while CDAI clinical remission and PRO clinical remission were achieved by 33.6% and 31.5% at W52, respectively. Endoscopic response increased from 30.2% at W12 to 40.3% at W52 among W12 PRO nonresponders (Table).
Discussion: Subcutaneous mirikizumab maintenance treatment over 40 weeks leads to incremental and sustained improvements in rates of clinical and endoscopic response regardless of PRO clinical response status after induction. A substantial proportion of clinical nonresponders at W12 improved early during maintenance treatment suggesting continued treatment with mirikizumab can be beneficial and lead to long-term response.

Figure: CDAI Clinical Remission and Endoscopic Response by W12 PRO Response Status
Disclosures:
Peter Bossuyt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Amgen – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Lecture fees. CAG – Lecture fees. Celltrion – Advisory Committee/Board Member, Lecture fees. CIRC – Advisory Committee/Board Member. Dr. Falk Pharma Benelux – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Lecture fees. EPGS – Lecture fees. Galapagos NV – Advisory Committee/Board Member, Lecture fees. Globalport – Lecture fees. Janssen – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Janssen – Lecture fees. Materia Prima – Lecture fees. Pentax – Advisory Committee/Board Member, Lecture fees. Pfizer – Grant/Research Support. PSI-CRO – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Scope – Lecture fees. Takeda – Advisory Committee/Board Member, Lecture fees. Tetrameros – Advisory Committee/Board Member. Viatris – Grant/Research Support.
David Laharie: AbbVie – Advisory Committee/Board Member, Consultant, Transport, Fees.. Alfasigma – Advisory Committee/Board Member, Consultant, Transport or Fees. Amgen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Celltrion – Advisory Committee/Board Member, Consultant, Transport, Fees.. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Transport, Fees.. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Transport, Fees.. Janssen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Medac – Advisory Committee/Board Member, Consultant, Transport or Fees. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Transport, Fees.. Pfizer – Advisory Committee/Board Member, Consultant, Transport, Fees.. Prometheus – Advisory Committee/Board Member, Consultant, Transport, Fees.. Takeda – Advisory Committee/Board Member, Consultant, Transport, Fees.. Theradiag – Advisory Committee/Board Member, Consultant, Transport, Fees..
Stephan Brand: Abbvie – Advisory Committee/Board Member, Speakers Bureau. BMS – Advisory Committee/Board Member. Celgene – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Falk Foundation – Speakers Bureau. Ferring – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. MSD – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Pierre Fabre – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Educational grant, Speakers Bureau. UCB – Advisory Committee/Board Member, Speakers Bureau. Vifor – Speakers Bureau.
Dominik Bettenworth: AbbVie – Advisory Committee/Board Member, Consultant. Alfasigma – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Arena – Advisory Committee/Board Member, Consultant. Art tempi – Advisory Committee/Board Member, Consultant. BNG Service GmbH – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. CED-Service GmbH – Advisory Committee/Board Member, Consultant. Celltrion – Advisory Committee/Board Member, Consultant. DGVS – Advisory Committee/Board Member, Consultant. Diaplan – Advisory Committee/Board Member, Consultant. Doctorflix – Advisory Committee/Board Member, Consultant. Else Kröner-Fresenius Foundation – Advisory Committee/Board Member, Consultant. Falk Foundation – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Fresenius – Advisory Committee/Board Member, Consultant. Galapagos – Advisory Committee/Board Member, Consultant. Gastro Today – Advisory Committee/Board Member, Consultant. GSK – Advisory Committee/Board Member, Consultant. Guidepoint – Advisory Committee/Board Member, Consultant. Hexal – Advisory Committee/Board Member, Consultant. Impulze – Advisory Committee/Board Member, Consultant. Janssen Cilag – Advisory Committee/Board Member, Consultant. Lilly – Advisory Committee/Board Member, Consultant. Medical Tribune – Advisory Committee/Board Member, Consultant. MedTriX – Advisory Committee/Board Member, Consultant. MSD – Advisory Committee/Board Member, Consultant. Mylan – Advisory Committee/Board Member, Consultant. Onkowissen – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Stada – Advisory Committee/Board Member, Consultant. StreamedUp – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Tetrameros – Advisory Committee/Board Member, Consultant. Thieme – Advisory Committee/Board Member, Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Biopiharma – Advisory Committee/Board Member, Consultant. Viatris – Advisory Committee/Board Member, Consultant. Vifor – Advisory Committee/Board Member, Consultant.
James Lindsey: AbbVie – Consultant, Grant/Research Support, Educational grant, Speakers Bureau. Amgen – Educational grant. Astra Zeneca – Consultant. BMS – Consultant, Educational grant. Celgene – Consultant. Celtrion – Consultant. Eli Lilly and Company – Consultant, Speakers Bureau. Engytix – Consultant. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Educational grant, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Educational grant. GSK – Consultant. Honoraria for developing and delivering the Cornerstones Health Best of DDW program – Educational grants. Janssen – Consultant, Educational grant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Educational grant, Speakers Bureau. Sandoz – Educational grant. Takeda – Consultant, Educational grant, Speakers Bureau. Teva – Consultant. Tillot’s – Educational grant, Speakers Bureau.
Sebastian Maier: Eli Lilly and Company – Employee, Stock Options.
Marijana Protic: Eli Lilly and Company – Employee, Stock Options.
Emily Hon: Eli Lilly and Company – Employee, Stock Options.
Hilde Carlier: Eli Lilly and Company – Employee, Stock Options.
Huaiyu Zang: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Walter Reinisch: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Actelion – Advisory Committee/Board Member, Consultant. Alpha Wasserman – Advisory Committee/Board Member, Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Cellerix – Advisory Committee/Board Member, Consultant. Cosmo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Grunenthal – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant. Millennium – Advisory Committee/Board Member, Consultant. Novo Nordisk – Advisory Committee/Board Member, Consultant. Nycomed – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Schering-Plough – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB Pharma – Advisory Committee/Board Member, Consultant. Vifor Pharma – Advisory Committee/Board Member, Consultant.
Peter Bossuyt, 1, David Laharie, 2, Stephan Brand, 3, Dominik Bettenworth, MD4, James Lindsey, PhD, BM, BCh5, Sebastian Maier, 6, Marijana Protic, 7, Emily Hon, 7, Hilde Carlier, 8, Huaiyu Zang, 9, Guanglei Yu, PhD10, Walter Reinisch, MD, PhD11. P1210 - Subcutaneous Mirikizumab Maintenance Treatment Over 40 Weeks Leads to Incremental Improvements in Rates of Clinical Remission and Endoscopic Response: Results From the Phase 3 VIVID-1 Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
