Sunday Poster Session
Category: IBD
P1205 - Assessing the Use of Topical Tacrolimus Therapy in Patients With an Ileal Pouch-Anal Anastomosis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Riley Smith, DO
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
Riley Smith, DO1, Joseph Carter Powers, MS, MD2, Mark Zemanek, DO1, Emma Dester, BS, MS3, Katherine Westbrook Cates, DO4, Shubha Bhat, PharmD1, Katherine Falloon, MD1, Benjamin L. Cohen, MD1, Taha Qazi, MD1
1Cleveland Clinic Foundation, Cleveland, OH; 2University of Michigan, Cleveland, OH; 3Cleveland Clinic Lerner College of Medicine, Cleveland, OH; 4Louisiana State University, New Orleans, LA
Introduction: Treatments for pouchitis and cuffitis in patients with an ileal pouch-anal anastomosis (IPAA) are similar to those for pre-surgical inflammatory bowel disease (IBD), but over 10% of patients still experience treatment failure. This highlights the need for novel therapies. Topical tacrolimus (suppositories or enemas) has shown early promise for proctitis, but data in IPAA patients are limited. This study aims to assess whether topical tacrolimus provides endoscopic and clinical benefits for patients with IBD and pouch and/or cuff inflammation.
Methods: This cohort study compared IBD patients with endoscopic pouch and/or cuff inflammation who used topical tacrolimus to controls who did not. Patients without follow-up pouchoscopy were excluded. The primary outcome was a composite of endoscopic improvement, defined as a ≥2-point decrease in the Pouch Disease Activity Index and improvement in mucosal breaks within 12 months. Secondary outcomes included clinical (symptomatic) improvement and localized endoscopic cuffitis improvement. Fisher’s exact test compared the proportion of patients in each group meeting outcomes.
Results: A total of 95 patients met eligibility criteria: 9 in the tacrolimus suppository cohort and 86 in the control group. The tacrolimus cohort had a shorter disease duration before colectomy and was more likely to use infliximab and upadacitinib post-operatively, but the groups were otherwise similar in demographics, clinical variables, and follow-up (Table 1). Overall, 48 (51.6%) patients showed endoscopic improvement of pouch and/or cuff inflammation within 12 months, with no significant difference between cohorts (71.1% vs. 50.0%; p = 0.437; Table 2). There were also no significant differences in improvement in clinical symptoms (p = 1.000) or rectal cuff inflammation (p = 0.401), though trends suggest a possible benefit of topical tacrolimus (Table 2).
Discussion: This study did not identify an endoscopic improvement, clinical improvement, or localized improvement of rectal cuff inflammation with the addition of novel topical tacrolimus therapy. There did appear to be a general trend toward endoscopic improvement with topical tacrolimus. However, due to our small sample size, the study was likely underpowered to detect smaller differences, despite trends suggesting a potential benefit in all outcomes. Future studies with larger cohorts are needed to explore trends and assess the full potential of topical tacrolimus.
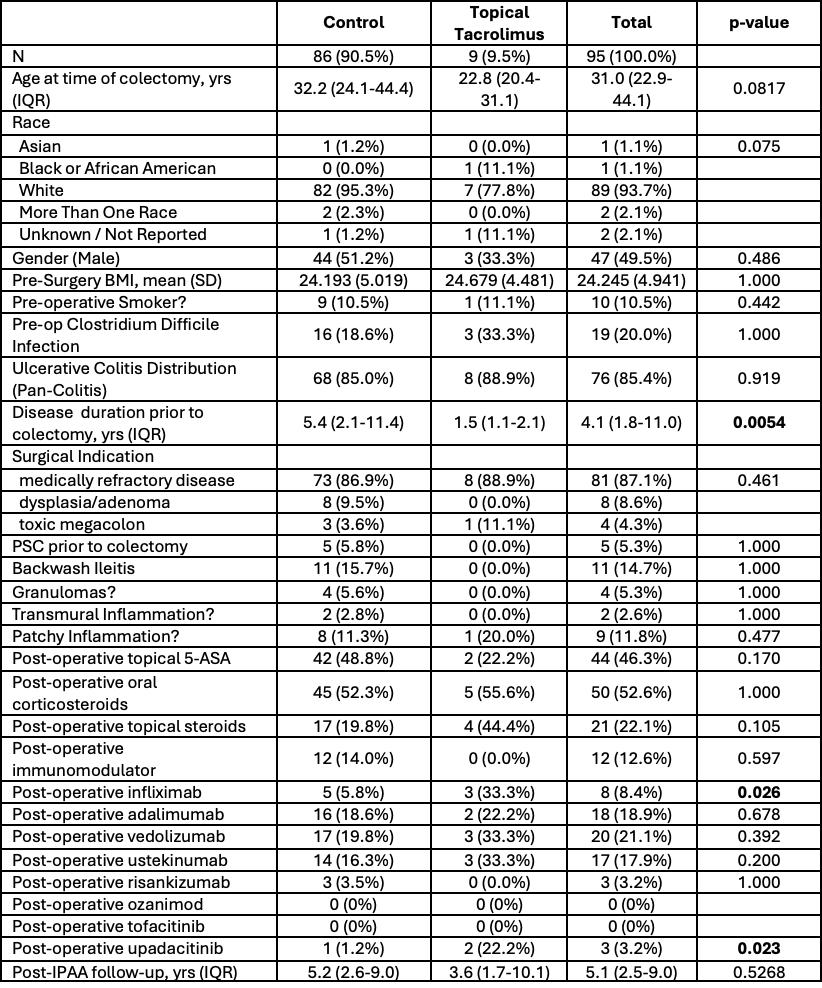
Figure: Table 1. Demographic and Clinical Characteristics of Patient Sample.
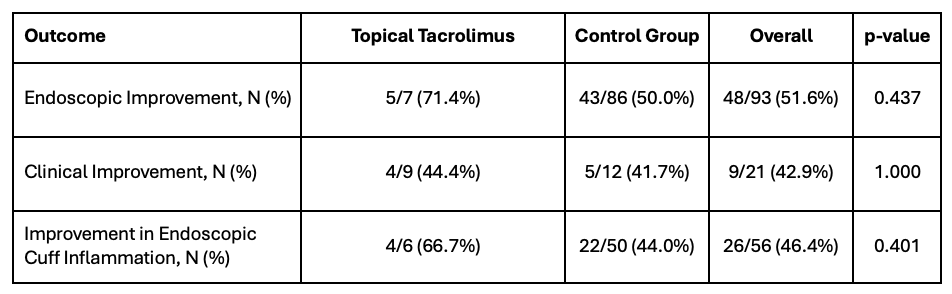
Figure: Table 2. Univariable Analysis of Study Outcomes. Proportion of patients in each cohort that met the outcome of interest were compared using Fisher’s exact test.
Disclosures:
Riley Smith indicated no relevant financial relationships.
Joseph Carter Powers indicated no relevant financial relationships.
Mark Zemanek indicated no relevant financial relationships.
Emma Dester indicated no relevant financial relationships.
Katherine Westbrook Cates indicated no relevant financial relationships.
Shubha Bhat: Celltrion – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Advisory Committee/Board Member.
Katherine Falloon: Cardinal Health, Inc. – Husband is employee. Janssen Pharmaceuticals, Inc. – Consultant. MD Education – Speaker. Pfizer – Grant/Research Support. Takeda Pharmaceuticals, Inc. – Speaker.
Benjamin Cohen: Abbvie – Advisory Committee/Board Member, Consultant, Speakers Bureau. ALPCO – Advisory Committee/Board Member, Consultant. Emmes Biopharma Services LLC – DSMB. J&J Innovative Medicine – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Consultant, Speakers Bureau.
Taha Qazi: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltirion – Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Johnson and Johnson – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. pfizer – Grant/Research Support.
Riley Smith, DO1, Joseph Carter Powers, MS, MD2, Mark Zemanek, DO1, Emma Dester, BS, MS3, Katherine Westbrook Cates, DO4, Shubha Bhat, PharmD1, Katherine Falloon, MD1, Benjamin L. Cohen, MD1, Taha Qazi, MD1. P1205 - Assessing the Use of Topical Tacrolimus Therapy in Patients With an Ileal Pouch-Anal Anastomosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic Foundation, Cleveland, OH; 2University of Michigan, Cleveland, OH; 3Cleveland Clinic Lerner College of Medicine, Cleveland, OH; 4Louisiana State University, New Orleans, LA
Introduction: Treatments for pouchitis and cuffitis in patients with an ileal pouch-anal anastomosis (IPAA) are similar to those for pre-surgical inflammatory bowel disease (IBD), but over 10% of patients still experience treatment failure. This highlights the need for novel therapies. Topical tacrolimus (suppositories or enemas) has shown early promise for proctitis, but data in IPAA patients are limited. This study aims to assess whether topical tacrolimus provides endoscopic and clinical benefits for patients with IBD and pouch and/or cuff inflammation.
Methods: This cohort study compared IBD patients with endoscopic pouch and/or cuff inflammation who used topical tacrolimus to controls who did not. Patients without follow-up pouchoscopy were excluded. The primary outcome was a composite of endoscopic improvement, defined as a ≥2-point decrease in the Pouch Disease Activity Index and improvement in mucosal breaks within 12 months. Secondary outcomes included clinical (symptomatic) improvement and localized endoscopic cuffitis improvement. Fisher’s exact test compared the proportion of patients in each group meeting outcomes.
Results: A total of 95 patients met eligibility criteria: 9 in the tacrolimus suppository cohort and 86 in the control group. The tacrolimus cohort had a shorter disease duration before colectomy and was more likely to use infliximab and upadacitinib post-operatively, but the groups were otherwise similar in demographics, clinical variables, and follow-up (Table 1). Overall, 48 (51.6%) patients showed endoscopic improvement of pouch and/or cuff inflammation within 12 months, with no significant difference between cohorts (71.1% vs. 50.0%; p = 0.437; Table 2). There were also no significant differences in improvement in clinical symptoms (p = 1.000) or rectal cuff inflammation (p = 0.401), though trends suggest a possible benefit of topical tacrolimus (Table 2).
Discussion: This study did not identify an endoscopic improvement, clinical improvement, or localized improvement of rectal cuff inflammation with the addition of novel topical tacrolimus therapy. There did appear to be a general trend toward endoscopic improvement with topical tacrolimus. However, due to our small sample size, the study was likely underpowered to detect smaller differences, despite trends suggesting a potential benefit in all outcomes. Future studies with larger cohorts are needed to explore trends and assess the full potential of topical tacrolimus.

Figure: Table 1. Demographic and Clinical Characteristics of Patient Sample.

Figure: Table 2. Univariable Analysis of Study Outcomes. Proportion of patients in each cohort that met the outcome of interest were compared using Fisher’s exact test.
Disclosures:
Riley Smith indicated no relevant financial relationships.
Joseph Carter Powers indicated no relevant financial relationships.
Mark Zemanek indicated no relevant financial relationships.
Emma Dester indicated no relevant financial relationships.
Katherine Westbrook Cates indicated no relevant financial relationships.
Shubha Bhat: Celltrion – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Advisory Committee/Board Member.
Katherine Falloon: Cardinal Health, Inc. – Husband is employee. Janssen Pharmaceuticals, Inc. – Consultant. MD Education – Speaker. Pfizer – Grant/Research Support. Takeda Pharmaceuticals, Inc. – Speaker.
Benjamin Cohen: Abbvie – Advisory Committee/Board Member, Consultant, Speakers Bureau. ALPCO – Advisory Committee/Board Member, Consultant. Emmes Biopharma Services LLC – DSMB. J&J Innovative Medicine – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Consultant, Speakers Bureau.
Taha Qazi: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltirion – Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Johnson and Johnson – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. pfizer – Grant/Research Support.
Riley Smith, DO1, Joseph Carter Powers, MS, MD2, Mark Zemanek, DO1, Emma Dester, BS, MS3, Katherine Westbrook Cates, DO4, Shubha Bhat, PharmD1, Katherine Falloon, MD1, Benjamin L. Cohen, MD1, Taha Qazi, MD1. P1205 - Assessing the Use of Topical Tacrolimus Therapy in Patients With an Ileal Pouch-Anal Anastomosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
