Sunday Poster Session
Category: IBD
P1201 - Outcomes of First-Line Anti-TNF Treatment in Ulcerative Proctitis Is Similar to Those with Left Sided Colitis: An Exploratory Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- PK
Polo Kostecki, DO (he/him/his)
Henry Ford Health
Warren, MI
Presenting Author(s)
Polo Kostecki, DO1, Renieh M. Nabaty, MD2, Jessica Jou, MD2, Jason JN. Schairer, MD2, Nirajan Budhathoki, PhD2, Najwa El-Nachef, MD2
1Henry Ford Health, Warren, MI; 2Henry Ford Health, Detroit, MI
Introduction: Ulcerative colitis (UC) is a chronic inflammatory bowel disease with variable disease distribution. Although anti-TNF (TNF) agents are well-established first-line therapies for moderate to severe UC, their role in the management of ulcerative proctitis (UP) is not well-described. Patients with UP are frequently excluded from clinical trials, limiting generalizability to this population. This study aimed to assess whether outcomes differ between patients with UP and left-sided colitis following initiation of TNF as first line advanced therapy.
Methods:
We conducted a retrospective cohort study of bionaive adult UC patients treated with TNF at a tertiary referral center between 2014 and 2023. Chart review confirmed disease extent at index colonoscopy and patients were stratified as having UP, defined as inflammation ≤20 cm from the anal verge, or left sided colitis, defined as inflammation extending >20cm from anal verge but not proximal to the mid transverse colon.
The primary outcome was a composite of clinical remission based on provider global assessment at 52 ± 4 weeks and drug persistence at 52 ± 4 weeks. Secondary outcomes included dose escalation of TNF, endoscopic response, biomarker trends, and adverse events. Baseline variables included age, sex, BMI, albumin, CRP, fecal calprotectin, and concurrent UC therapies. Categorical data were compared by Chi-square tests or exact tests. Continuous data were compared by Wilcoxon rank sum tests. Logistic regression was conducted to test disease location effect on the outcome of interest adjusting for the baseline covariates.
Results: Fifty patients met inclusion criteria (26 UP, 24 left-sided). Rates of the primary outcome were identical across groups (50%, p > 0.900). No significant differences were observed in dose escalations (42% proctitis vs. 33% left-sided, p = 0.500), endoscopic response, or change in fecal calprotectin. Albumin was lower in the left-sided group (median 3.5 vs. 4.05 g/dL, p < 0.001), but this did not correlate with outcomes. Multivariable analysis adjusting for baseline factors showed no significant association between disease location and treatment response.
Discussion: Patients with UP demonstrated comparable outcomes to those with left-sided colitis when treated with first-line TNF therapy in this exploratory study. These findings challenge the rationale for excluding UP patients from clinical trials and support their inclusion in future studies and treatment guidelines.
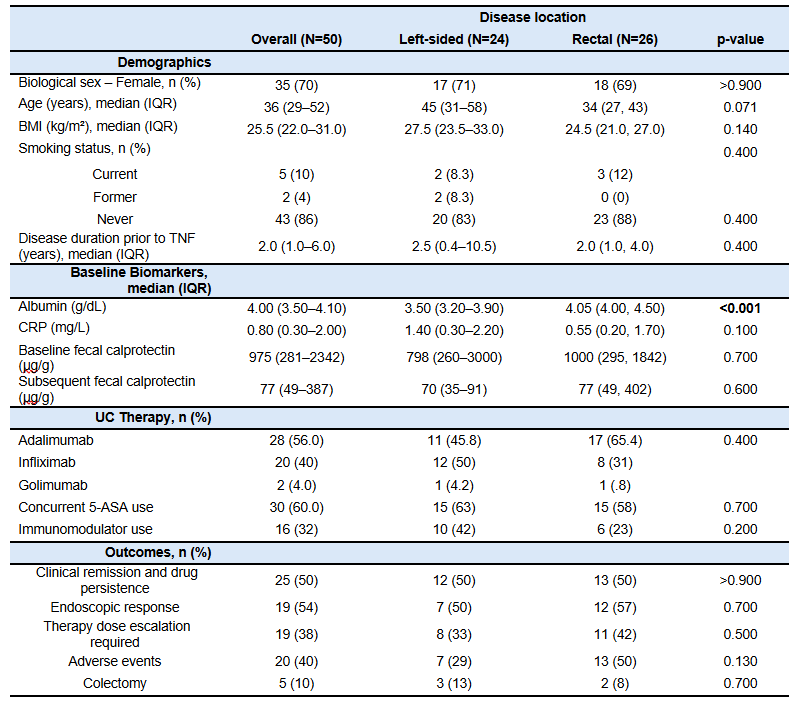
Figure: Table 1. Baseline characteristics, treatment details, and clinical outcomes of patients with ulcerative colitis, stratified by disease location. Baseline albumin levels were significantly lower in patients with left-sided colitis compared to those with rectal involvement (p < 0.001). Outcomes were evaluated at 52 ±4 weeks, but colectomy was reported as a historical event and not limited to the 52-week assessment window.
Disclosures:
Polo Kostecki indicated no relevant financial relationships.
Renieh Nabaty indicated no relevant financial relationships.
Jessica Jou indicated no relevant financial relationships.
Jason Schairer indicated no relevant financial relationships.
Nirajan Budhathoki indicated no relevant financial relationships.
Najwa El-Nachef: Abbvie – Grant/Research Support. Abivax – Grant/Research Support. Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Polo Kostecki, DO1, Renieh M. Nabaty, MD2, Jessica Jou, MD2, Jason JN. Schairer, MD2, Nirajan Budhathoki, PhD2, Najwa El-Nachef, MD2. P1201 - Outcomes of First-Line Anti-TNF Treatment in Ulcerative Proctitis Is Similar to Those with Left Sided Colitis: An Exploratory Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Henry Ford Health, Warren, MI; 2Henry Ford Health, Detroit, MI
Introduction: Ulcerative colitis (UC) is a chronic inflammatory bowel disease with variable disease distribution. Although anti-TNF (TNF) agents are well-established first-line therapies for moderate to severe UC, their role in the management of ulcerative proctitis (UP) is not well-described. Patients with UP are frequently excluded from clinical trials, limiting generalizability to this population. This study aimed to assess whether outcomes differ between patients with UP and left-sided colitis following initiation of TNF as first line advanced therapy.
Methods:
We conducted a retrospective cohort study of bionaive adult UC patients treated with TNF at a tertiary referral center between 2014 and 2023. Chart review confirmed disease extent at index colonoscopy and patients were stratified as having UP, defined as inflammation ≤20 cm from the anal verge, or left sided colitis, defined as inflammation extending >20cm from anal verge but not proximal to the mid transverse colon.
The primary outcome was a composite of clinical remission based on provider global assessment at 52 ± 4 weeks and drug persistence at 52 ± 4 weeks. Secondary outcomes included dose escalation of TNF, endoscopic response, biomarker trends, and adverse events. Baseline variables included age, sex, BMI, albumin, CRP, fecal calprotectin, and concurrent UC therapies. Categorical data were compared by Chi-square tests or exact tests. Continuous data were compared by Wilcoxon rank sum tests. Logistic regression was conducted to test disease location effect on the outcome of interest adjusting for the baseline covariates.
Results: Fifty patients met inclusion criteria (26 UP, 24 left-sided). Rates of the primary outcome were identical across groups (50%, p > 0.900). No significant differences were observed in dose escalations (42% proctitis vs. 33% left-sided, p = 0.500), endoscopic response, or change in fecal calprotectin. Albumin was lower in the left-sided group (median 3.5 vs. 4.05 g/dL, p < 0.001), but this did not correlate with outcomes. Multivariable analysis adjusting for baseline factors showed no significant association between disease location and treatment response.
Discussion: Patients with UP demonstrated comparable outcomes to those with left-sided colitis when treated with first-line TNF therapy in this exploratory study. These findings challenge the rationale for excluding UP patients from clinical trials and support their inclusion in future studies and treatment guidelines.

Figure: Table 1. Baseline characteristics, treatment details, and clinical outcomes of patients with ulcerative colitis, stratified by disease location. Baseline albumin levels were significantly lower in patients with left-sided colitis compared to those with rectal involvement (p < 0.001). Outcomes were evaluated at 52 ±4 weeks, but colectomy was reported as a historical event and not limited to the 52-week assessment window.
Disclosures:
Polo Kostecki indicated no relevant financial relationships.
Renieh Nabaty indicated no relevant financial relationships.
Jessica Jou indicated no relevant financial relationships.
Jason Schairer indicated no relevant financial relationships.
Nirajan Budhathoki indicated no relevant financial relationships.
Najwa El-Nachef: Abbvie – Grant/Research Support. Abivax – Grant/Research Support. Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Polo Kostecki, DO1, Renieh M. Nabaty, MD2, Jessica Jou, MD2, Jason JN. Schairer, MD2, Nirajan Budhathoki, PhD2, Najwa El-Nachef, MD2. P1201 - Outcomes of First-Line Anti-TNF Treatment in Ulcerative Proctitis Is Similar to Those with Left Sided Colitis: An Exploratory Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

