Sunday Poster Session
Category: IBD
P1189 - Mirikizumab Demonstrates Long-term Efficacy on Histological Resolution in the Phase 3, Multicenter, Open-Label VIVID-2 Extension Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Vipul Jairath, MBChB, DPhil, MRCP
Department of Medicine and Department of Epidemiology and Biostatistics, Western University
London, ON, Canada
Presenting Author(s)
Walter Reinisch, MD, PhD1, Vipul Jairath, MBChB, DPhil, MRCP2, Marijana Protic, 3, Gert De Hertogh, 4, Noam Harpaz, 5, Tadakazu Hisamatsu, MD, PhD6, Geert R. D’Haens, MD, PhD7, Rish Pai, 8, Nathan Morris, 3, Rebecca Hozak, 3, Guanglei Yu, PhD9, Frederick Durand, 3, Fernando Magro, MD, PhD10
1Medical University of Vienna, Department of Internal Medicine III, Division of Gastroenterology and Hepatology, Spitalgasse, Wien, Austria; 2Department of Medicine and Department of Epidemiology and Biostatistics, Western University, London, ON, Canada; 3Eli Lilly and Company, Indianapolis, IN; 4Faculty of Medicine, KU, Leuven, Leuven, Brabant Wallon, Belgium; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 7Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 8Mayo Clinic, Scottsdale, AZ; 9Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 10University of Porto, Porto, Porto, Portugal
Introduction: VIVID-1 evaluated systematically obtained biopsies from 5 intestinal segments demonstrating that histologic (histo) readouts were impacted upon treatment with mirikizumab (MIRI).
Methods: VIVID-2, the long-term extension study of VIVID-1, examines histo and combined endoscopic(endo)-histo endpoints at Week(W)104. These data are presented, as is the impact of 1-year(yr) histo response (H-res) and histo remission (H-rem) on endoscopic and clinical outcomes after 2 yrs of MIRI treatment. In VIVID-1, the MIRI group received induction with 900 mg intravenously (IV) at W0, 4, and 8, then 300 mg subcutaneously (SC) every 4W. W52 patients (pts) in endo response (E-res; ≥50% reduction from baseline in Simple Endoscopic Score for CD) continued the same MIRI SC dosing in VIVID-2. Endo non-responders received reinduction with MIRI IV at the start of VIVID-2 followed by SC dosing (IV-SC) as described. Two specimens from each of 5 intestinal segments (1 ileal, 4 colonic) were obtained from the edge of ulcers, or the most inflamed mucosa from randomized pts at screening, W12, W52 (VIVID-1), and W104 (W52 of VIVID-2). H-res: absence of epithelial neutrophils and epithelial damage, erosions, and ulceration or ≥50% decrease in either the active Robarts Histopathology Index or the active Global Histology Activity Score. H-rem: absence of mucosal neutrophils, no epithelial damage, and no erosions and ulcers. The criteria for H-res and H-rem had to be met in all 5 segments.
Results: Among pts in E-res at W52 of VIVID-1: 81.2% maintained H-res and 57.5% maintained H-rem after an additional 52 weeks of MIRI SC treatment (Table 1A), 70.8% maintained combined endo-histo response. Interestingly, 25.6% pts who were in H-Res, but not in E-res at W52 of VIVID-1 maintained H-res and gained E-res during the 2nd year of MIRI treatment (IV-SC). In the overall MIRI-treated population (SC-SC and IV-SC), both H-res and H-rem were significantly associated with E-res (p< 0.001) and endo-remission (p< 0.001) at W104 while associations with Crohn’s Disease Activity Index remission (p=0.313 and p=0.771, respectively) and bowel urgency remission (p=0.122 and p=0.517, respectively) were not identified (Table 1B).
Discussion: In VIVID-2, achievement of histologic response and remission after 1 yr of miri treatment was strongly associated with longer term endoscopic outcomes. High rates of histologic and combined endo-histo endpoints were maintained after 2 yrs of treatment.
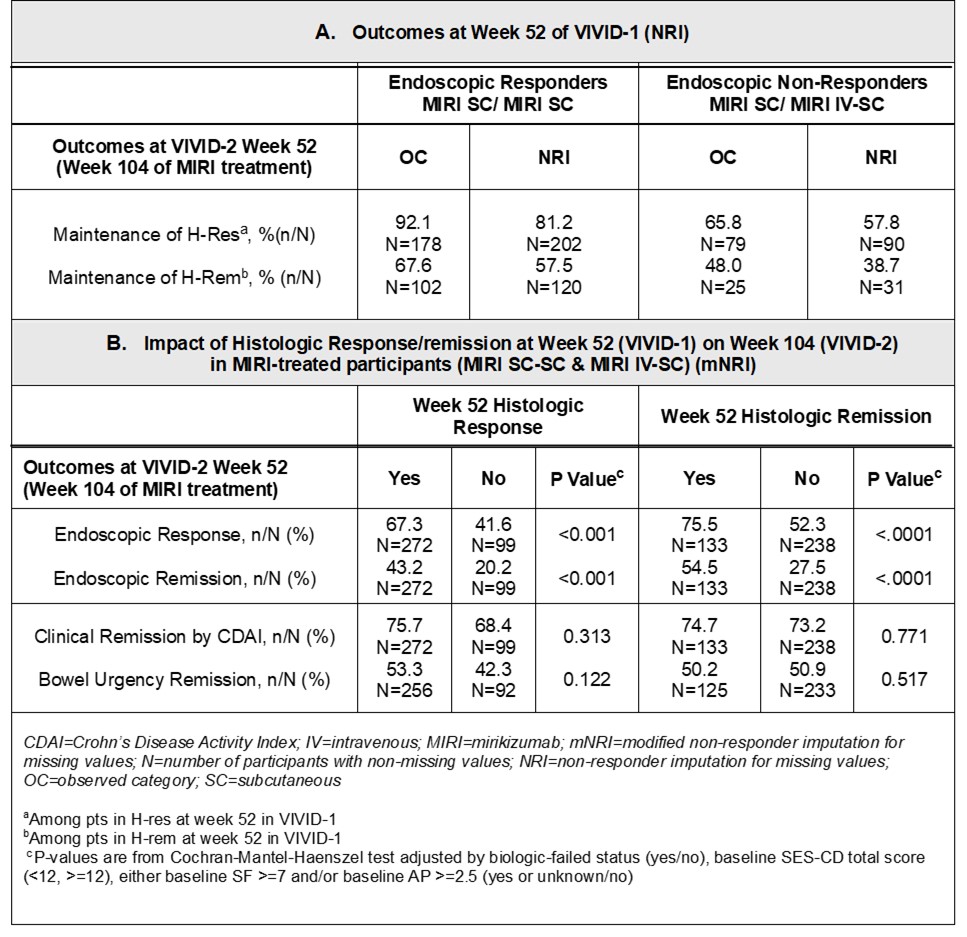
Figure: Week(W) 52 Outcomes and the Impact of Histologic Response/Remission at W52 on W104 in the Overall MIRI-treated Patients
Disclosures:
Walter Reinisch: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Actelion – Advisory Committee/Board Member, Consultant. Alpha Wasserman – Advisory Committee/Board Member, Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Cellerix – Advisory Committee/Board Member, Consultant. Cosmo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Grunenthal – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant. Millennium – Advisory Committee/Board Member, Consultant. Novo Nordisk – Advisory Committee/Board Member, Consultant. Nycomed – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Schering-Plough – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB Pharma – Advisory Committee/Board Member, Consultant. Vifor Pharma – Advisory Committee/Board Member, Consultant.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Consultant. Enthera – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Consultant. JAMP – Consultant. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Syndegen – Consultant. Takeda – Consultant, Intellectual Property/Patents, Speakers Bureau. TD Securities – Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Marijana Protic: Eli Lilly and Company – Employee, Stock Options.
Gert De Hertogh: Centocor Inc – Fees for clinical trial activities. Eli Lilly and Company – Consultant. MRM Health – Consultant.
Noam Harpaz: AbbVie – Advisory Committee/Board Member, Consultant. Bristol Meyers Squibb – Advisory Committee/Board Member, Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. PathAI – Advisory Committee/Board Member, Consultant.
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Rish Pai: Alimentiv – Consultant. Allergan – Consultant. Bracco – Consultant.
Nathan Morris: Eli Lilly and Company – Employee, Stock Options.
Rebecca Hozak: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Fernando Magro: AbbVie – Speakers Bureau. Arena Pharmaceuticals – Speakers Bureau. Biogen – Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring Pharmaceuticals – Speakers Bureau. Hospira – Speakers Bureau. Janssen – Speakers Bureau. Laboratórios Vitoria – Speakers Bureau. Merck Sharp & Dohme – Speakers Bureau. Pfizer – Speakers Bureau. Sandoz – Speakers Bureau. Takeda – Speakers Bureau. UCB Pharma – Speakers Bureau. Vifor Pharma – Speakers Bureau.
Walter Reinisch, MD, PhD1, Vipul Jairath, MBChB, DPhil, MRCP2, Marijana Protic, 3, Gert De Hertogh, 4, Noam Harpaz, 5, Tadakazu Hisamatsu, MD, PhD6, Geert R. D’Haens, MD, PhD7, Rish Pai, 8, Nathan Morris, 3, Rebecca Hozak, 3, Guanglei Yu, PhD9, Frederick Durand, 3, Fernando Magro, MD, PhD10. P1189 - Mirikizumab Demonstrates Long-term Efficacy on Histological Resolution in the Phase 3, Multicenter, Open-Label VIVID-2 Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Medical University of Vienna, Department of Internal Medicine III, Division of Gastroenterology and Hepatology, Spitalgasse, Wien, Austria; 2Department of Medicine and Department of Epidemiology and Biostatistics, Western University, London, ON, Canada; 3Eli Lilly and Company, Indianapolis, IN; 4Faculty of Medicine, KU, Leuven, Leuven, Brabant Wallon, Belgium; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 7Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 8Mayo Clinic, Scottsdale, AZ; 9Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 10University of Porto, Porto, Porto, Portugal
Introduction: VIVID-1 evaluated systematically obtained biopsies from 5 intestinal segments demonstrating that histologic (histo) readouts were impacted upon treatment with mirikizumab (MIRI).
Methods: VIVID-2, the long-term extension study of VIVID-1, examines histo and combined endoscopic(endo)-histo endpoints at Week(W)104. These data are presented, as is the impact of 1-year(yr) histo response (H-res) and histo remission (H-rem) on endoscopic and clinical outcomes after 2 yrs of MIRI treatment. In VIVID-1, the MIRI group received induction with 900 mg intravenously (IV) at W0, 4, and 8, then 300 mg subcutaneously (SC) every 4W. W52 patients (pts) in endo response (E-res; ≥50% reduction from baseline in Simple Endoscopic Score for CD) continued the same MIRI SC dosing in VIVID-2. Endo non-responders received reinduction with MIRI IV at the start of VIVID-2 followed by SC dosing (IV-SC) as described. Two specimens from each of 5 intestinal segments (1 ileal, 4 colonic) were obtained from the edge of ulcers, or the most inflamed mucosa from randomized pts at screening, W12, W52 (VIVID-1), and W104 (W52 of VIVID-2). H-res: absence of epithelial neutrophils and epithelial damage, erosions, and ulceration or ≥50% decrease in either the active Robarts Histopathology Index or the active Global Histology Activity Score. H-rem: absence of mucosal neutrophils, no epithelial damage, and no erosions and ulcers. The criteria for H-res and H-rem had to be met in all 5 segments.
Results: Among pts in E-res at W52 of VIVID-1: 81.2% maintained H-res and 57.5% maintained H-rem after an additional 52 weeks of MIRI SC treatment (Table 1A), 70.8% maintained combined endo-histo response. Interestingly, 25.6% pts who were in H-Res, but not in E-res at W52 of VIVID-1 maintained H-res and gained E-res during the 2nd year of MIRI treatment (IV-SC). In the overall MIRI-treated population (SC-SC and IV-SC), both H-res and H-rem were significantly associated with E-res (p< 0.001) and endo-remission (p< 0.001) at W104 while associations with Crohn’s Disease Activity Index remission (p=0.313 and p=0.771, respectively) and bowel urgency remission (p=0.122 and p=0.517, respectively) were not identified (Table 1B).
Discussion: In VIVID-2, achievement of histologic response and remission after 1 yr of miri treatment was strongly associated with longer term endoscopic outcomes. High rates of histologic and combined endo-histo endpoints were maintained after 2 yrs of treatment.

Figure: Week(W) 52 Outcomes and the Impact of Histologic Response/Remission at W52 on W104 in the Overall MIRI-treated Patients
Disclosures:
Walter Reinisch: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Actelion – Advisory Committee/Board Member, Consultant. Alpha Wasserman – Advisory Committee/Board Member, Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Cellerix – Advisory Committee/Board Member, Consultant. Cosmo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Grunenthal – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant. Millennium – Advisory Committee/Board Member, Consultant. Novo Nordisk – Advisory Committee/Board Member, Consultant. Nycomed – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Schering-Plough – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB Pharma – Advisory Committee/Board Member, Consultant. Vifor Pharma – Advisory Committee/Board Member, Consultant.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Consultant. Enthera – Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Consultant. JAMP – Consultant. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Syndegen – Consultant. Takeda – Consultant, Intellectual Property/Patents, Speakers Bureau. TD Securities – Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Marijana Protic: Eli Lilly and Company – Employee, Stock Options.
Gert De Hertogh: Centocor Inc – Fees for clinical trial activities. Eli Lilly and Company – Consultant. MRM Health – Consultant.
Noam Harpaz: AbbVie – Advisory Committee/Board Member, Consultant. Bristol Meyers Squibb – Advisory Committee/Board Member, Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. PathAI – Advisory Committee/Board Member, Consultant.
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Rish Pai: Alimentiv – Consultant. Allergan – Consultant. Bracco – Consultant.
Nathan Morris: Eli Lilly and Company – Employee, Stock Options.
Rebecca Hozak: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Fernando Magro: AbbVie – Speakers Bureau. Arena Pharmaceuticals – Speakers Bureau. Biogen – Speakers Bureau. Bristol Myers Squibb – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring Pharmaceuticals – Speakers Bureau. Hospira – Speakers Bureau. Janssen – Speakers Bureau. Laboratórios Vitoria – Speakers Bureau. Merck Sharp & Dohme – Speakers Bureau. Pfizer – Speakers Bureau. Sandoz – Speakers Bureau. Takeda – Speakers Bureau. UCB Pharma – Speakers Bureau. Vifor Pharma – Speakers Bureau.
Walter Reinisch, MD, PhD1, Vipul Jairath, MBChB, DPhil, MRCP2, Marijana Protic, 3, Gert De Hertogh, 4, Noam Harpaz, 5, Tadakazu Hisamatsu, MD, PhD6, Geert R. D’Haens, MD, PhD7, Rish Pai, 8, Nathan Morris, 3, Rebecca Hozak, 3, Guanglei Yu, PhD9, Frederick Durand, 3, Fernando Magro, MD, PhD10. P1189 - Mirikizumab Demonstrates Long-term Efficacy on Histological Resolution in the Phase 3, Multicenter, Open-Label VIVID-2 Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
