Sunday Poster Session
Category: IBD
P1162 - Effectiveness of Switching From Intravenous to Subcutaneous Infliximab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Pranay T. Vaghela, MBBS (he/him/his)
The Maharaja Sayajirao University of Baroda
Navsari, Gujarat, India
Presenting Author(s)
Jyoti Yadav, MBBS1, Rohan Raj, MBBS2, Pranay T. Vaghela, MBBS3, Jithin Kolli, MBBS4, Yousuf Hassan Syed, MD5, Nandini Hemant. Patel, MBBS6, Hari Priya Nistala, MBBS7, Rupak Desai, MBBS8
1JSS Medical college, Mysore, Karnataka, India; 2Memorial Hospital at Gulfport, Gulfport, MS; 3The Maharaja Sayajirao University of Baroda, Navsari, Gujarat, India; 4JSS MEDICAL COLLEGE, Andra Pradesh, Andhra Pradesh, India; 5Team Health, Las Vegas, NV; 6Pramukhswami Medical College, Gujarat, India, Anand, Gujarat, India; 7Maharajah's Institute of Medical Sciences, Leander, TX; 8Independent Outcomes Researcher, Atlanta, GA
Introduction: Intravenous (IV) infliximab is widely used in managing inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis. Recently, subcutaneous (SC) infliximab has gained attention as a maintenance strategy, offering improved patient convenience, enhanced pharmacokinetics, and reduced healthcare burden. With growing real-world and trial-based evidence, a comprehensive synthesis is needed to guide therapeutic decisions. This study systematically reviews outcomes following the switch from IV to SC infliximab in IBD patients.
Methods: We conducted a systematic review and meta-analysis in accordance with PRISMA guidelines to evaluate 12-month clinical and biochemical remission, as well as treatment discontinuation rates, after switching from IV to SC infliximab in ulcerative colitis and Crohn’s disease. Eight eligible studies were identified through PubMed, Scopus, and Google Scholar searches up to April 2025. These studies were conducted across multiple nations. Two reviewers independently extracted data and assessed study quality using the Newcastle–Ottawa Scale. Pooled estimates were calculated using a random-effects model. Heterogeneity was assessed using the I² statistic, and leave-one-out sensitivity analyses were performed to test robustness.
Results: Across the eight studies comprising 1,076 patients, Crohn’s disease was the predominant subtype in most cohorts, with males representing a greater proportion of participants in several studies. Twelve-month clinical remission was achieved in 80.8% of patients (95% CI: 77.2–84.4), with moderate heterogeneity (I² = 42.26%), suggesting consistent benefit across studies. Biochemical remission was observed in 61.3% (95% CI: 55.1–67.5), also with moderate heterogeneity (I² = 49.29). The overall discontinuation rate was low at 7.2% (95% CI: 3.6–10.7), though substantial heterogeneity was noted (I² = 83.5), indicating variability in treatment persistence or tolerability.
Discussion: These results support the effectiveness and feasibility of switching to subcutaneous infliximab in maintaining remission over one year, with acceptable discontinuation rates. More uniform study designs may help clarify the determinants of treatment discontinuation.
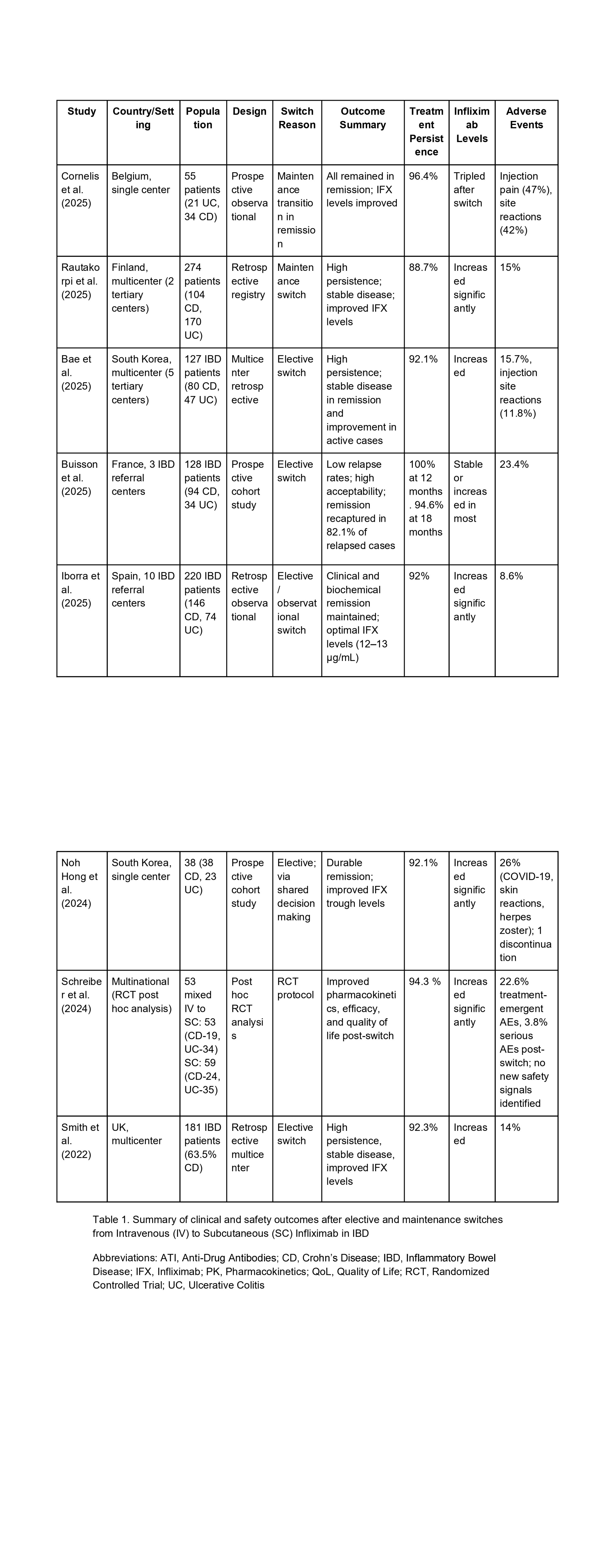
Figure: Table 1. Summary of studies evaluating 12-month outcomes after switching from intravenous to subcutaneous infliximab in inflammatory bowel disease.
Disclosures:
Jyoti Yadav indicated no relevant financial relationships.
Rohan Raj indicated no relevant financial relationships.
Pranay Vaghela indicated no relevant financial relationships.
Jithin Kolli indicated no relevant financial relationships.
Yousuf Hassan Syed indicated no relevant financial relationships.
Nandini Patel indicated no relevant financial relationships.
Hari Priya Nistala indicated no relevant financial relationships.
Rupak Desai indicated no relevant financial relationships.
Jyoti Yadav, MBBS1, Rohan Raj, MBBS2, Pranay T. Vaghela, MBBS3, Jithin Kolli, MBBS4, Yousuf Hassan Syed, MD5, Nandini Hemant. Patel, MBBS6, Hari Priya Nistala, MBBS7, Rupak Desai, MBBS8. P1162 - Effectiveness of Switching From Intravenous to Subcutaneous Infliximab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1JSS Medical college, Mysore, Karnataka, India; 2Memorial Hospital at Gulfport, Gulfport, MS; 3The Maharaja Sayajirao University of Baroda, Navsari, Gujarat, India; 4JSS MEDICAL COLLEGE, Andra Pradesh, Andhra Pradesh, India; 5Team Health, Las Vegas, NV; 6Pramukhswami Medical College, Gujarat, India, Anand, Gujarat, India; 7Maharajah's Institute of Medical Sciences, Leander, TX; 8Independent Outcomes Researcher, Atlanta, GA
Introduction: Intravenous (IV) infliximab is widely used in managing inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis. Recently, subcutaneous (SC) infliximab has gained attention as a maintenance strategy, offering improved patient convenience, enhanced pharmacokinetics, and reduced healthcare burden. With growing real-world and trial-based evidence, a comprehensive synthesis is needed to guide therapeutic decisions. This study systematically reviews outcomes following the switch from IV to SC infliximab in IBD patients.
Methods: We conducted a systematic review and meta-analysis in accordance with PRISMA guidelines to evaluate 12-month clinical and biochemical remission, as well as treatment discontinuation rates, after switching from IV to SC infliximab in ulcerative colitis and Crohn’s disease. Eight eligible studies were identified through PubMed, Scopus, and Google Scholar searches up to April 2025. These studies were conducted across multiple nations. Two reviewers independently extracted data and assessed study quality using the Newcastle–Ottawa Scale. Pooled estimates were calculated using a random-effects model. Heterogeneity was assessed using the I² statistic, and leave-one-out sensitivity analyses were performed to test robustness.
Results: Across the eight studies comprising 1,076 patients, Crohn’s disease was the predominant subtype in most cohorts, with males representing a greater proportion of participants in several studies. Twelve-month clinical remission was achieved in 80.8% of patients (95% CI: 77.2–84.4), with moderate heterogeneity (I² = 42.26%), suggesting consistent benefit across studies. Biochemical remission was observed in 61.3% (95% CI: 55.1–67.5), also with moderate heterogeneity (I² = 49.29). The overall discontinuation rate was low at 7.2% (95% CI: 3.6–10.7), though substantial heterogeneity was noted (I² = 83.5), indicating variability in treatment persistence or tolerability.
Discussion: These results support the effectiveness and feasibility of switching to subcutaneous infliximab in maintaining remission over one year, with acceptable discontinuation rates. More uniform study designs may help clarify the determinants of treatment discontinuation.

Figure: Table 1. Summary of studies evaluating 12-month outcomes after switching from intravenous to subcutaneous infliximab in inflammatory bowel disease.
Disclosures:
Jyoti Yadav indicated no relevant financial relationships.
Rohan Raj indicated no relevant financial relationships.
Pranay Vaghela indicated no relevant financial relationships.
Jithin Kolli indicated no relevant financial relationships.
Yousuf Hassan Syed indicated no relevant financial relationships.
Nandini Patel indicated no relevant financial relationships.
Hari Priya Nistala indicated no relevant financial relationships.
Rupak Desai indicated no relevant financial relationships.
Jyoti Yadav, MBBS1, Rohan Raj, MBBS2, Pranay T. Vaghela, MBBS3, Jithin Kolli, MBBS4, Yousuf Hassan Syed, MD5, Nandini Hemant. Patel, MBBS6, Hari Priya Nistala, MBBS7, Rupak Desai, MBBS8. P1162 - Effectiveness of Switching From Intravenous to Subcutaneous Infliximab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
