Sunday Poster Session
Category: IBD
P1161 - Comparing the Safety of Risankizumab vs Other Biological Therapies in Older Adults With Crohn’s Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- AB
Anthony Bilotta, II, MD, PhD (he/him/his)
University of Massachusetts Chan Medical School
Worcester, MA
Presenting Author(s)
Anthony Bilotta, MD, PhD1, Abbas Rupawala, MD2
1University of Massachusetts Chan Medical School, Worcester, MA; 2UMass Chan Medical School, Worcester, MA
Introduction: Adults older than 60 years old are one of the fastest growing populations of patients with Crohn’s disease (CD). Despite this, the efficacy and safety of newer biological therapies on this population remain understudied. The aim of our study is to examine the safety of risankizumab as compared to other approved biologic therapies for CD in patients 60 years and older.
Methods: We performed a retrospective cohort study using data from the TriNetX Research Network (Cambridge, MA). We identified patients 60 years and older with CD who were on risankizumab, anti-tumor necrosis factor (anti-TNFs), ustekinumab, or vedolizumab. Three separate analyses were performed to compare the risks of major adverse cardiac events (MACE), thrombosis, infection, malignancy and healthcare utilization in patients on risankizumab as compared anti-TNFs, ustekinumab, or vedolizumab. The dates of the study were between 6/17/2022 to 3/30/2025. 1:1 propensity score matching (PSM) was performed for age at index, demographics, glucocorticoid use, disease extent, healthcare utilization, laboratory values, inflammatory markers, and comorbidities. Outcomes were assessed between 31 days and one year of therapy initiation. Outcomes are reported as hazard ratios (HR) with 95% confidence intervals (CI).
Results: After PSM, there were 670 patients in each of the risankizumab and anti-TNF cohorts (mean age: 68.1 ± 6.4 vs 68.2 ± 6.1 years old), 608 patients in each of the risankizumab and ustekinumab cohorts (mean age: 67.8 ± 5.9 vs 67.9 ± 6 years old) and 552 patients in each of the risankizumab and vedolizumab cohorts (mean age: 69.1 ± 6.6 vs 68.9 ± 6.2 years old). There were no significant differences in any baseline characteristics. Patients on anti-TNFs were more likely to have pneumonia (HR: 2.22; 95%CI: 1.17-4.20), and sepsis (HR: 2.48; 95%CI: 1.05-5.87) as compared to those on risankizumab. Patients on ustekinumab were more likely to have sepsis (HR: 4.36; 95%CI: 1.27-14.90) as compared to patients on risankizumab. There were no differences in any adverse events between risankizumab and vedolizumab (Figure 1).
Discussion: In this retrospective cohort study in patients 60 years or older with CD, there were no differences in adverse events between patients on risankizumab and vedolizumab. Compared to risankizumab, patients on ustekinumab or anti-TNFs had a higher likelihood of infection. This suggest that risankizumab or vedolizumab may be safer options for older adults with CD.
Forest plots generated by ChatGPT.
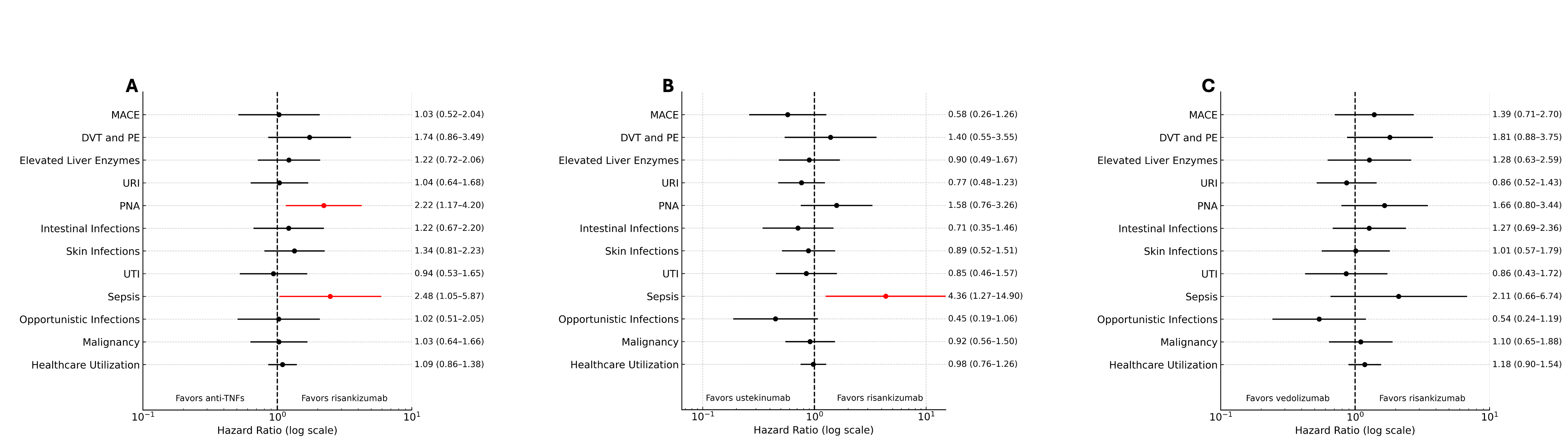
Figure: Figure 1: Forest plots of adverse events between 31 days and one year of therapy initiation in patients on risankizumab as compared to anti-TNFs (A), ustekinumab (B), and vedolizumab (C). Major adverse cardiac events (MACE) include myocardial infarction, transient ischemic attacks, and cerebral infarction. DVT: deep vein thrombosis; PE: pulmonary embolism; PNA: pneumonia; URI: upper respiratory tract infection; UTI: urinary tract infection. Forest plots were generated by ChatGPT.
Disclosures:
Anthony Bilotta indicated no relevant financial relationships.
Abbas Rupawala: Abbvie – Consultant, Speakers Bureau. Pfizer – Consultant. Takeda – Consultant.
Anthony Bilotta, MD, PhD1, Abbas Rupawala, MD2. P1161 - Comparing the Safety of Risankizumab vs Other Biological Therapies in Older Adults With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Massachusetts Chan Medical School, Worcester, MA; 2UMass Chan Medical School, Worcester, MA
Introduction: Adults older than 60 years old are one of the fastest growing populations of patients with Crohn’s disease (CD). Despite this, the efficacy and safety of newer biological therapies on this population remain understudied. The aim of our study is to examine the safety of risankizumab as compared to other approved biologic therapies for CD in patients 60 years and older.
Methods: We performed a retrospective cohort study using data from the TriNetX Research Network (Cambridge, MA). We identified patients 60 years and older with CD who were on risankizumab, anti-tumor necrosis factor (anti-TNFs), ustekinumab, or vedolizumab. Three separate analyses were performed to compare the risks of major adverse cardiac events (MACE), thrombosis, infection, malignancy and healthcare utilization in patients on risankizumab as compared anti-TNFs, ustekinumab, or vedolizumab. The dates of the study were between 6/17/2022 to 3/30/2025. 1:1 propensity score matching (PSM) was performed for age at index, demographics, glucocorticoid use, disease extent, healthcare utilization, laboratory values, inflammatory markers, and comorbidities. Outcomes were assessed between 31 days and one year of therapy initiation. Outcomes are reported as hazard ratios (HR) with 95% confidence intervals (CI).
Results: After PSM, there were 670 patients in each of the risankizumab and anti-TNF cohorts (mean age: 68.1 ± 6.4 vs 68.2 ± 6.1 years old), 608 patients in each of the risankizumab and ustekinumab cohorts (mean age: 67.8 ± 5.9 vs 67.9 ± 6 years old) and 552 patients in each of the risankizumab and vedolizumab cohorts (mean age: 69.1 ± 6.6 vs 68.9 ± 6.2 years old). There were no significant differences in any baseline characteristics. Patients on anti-TNFs were more likely to have pneumonia (HR: 2.22; 95%CI: 1.17-4.20), and sepsis (HR: 2.48; 95%CI: 1.05-5.87) as compared to those on risankizumab. Patients on ustekinumab were more likely to have sepsis (HR: 4.36; 95%CI: 1.27-14.90) as compared to patients on risankizumab. There were no differences in any adverse events between risankizumab and vedolizumab (Figure 1).
Discussion: In this retrospective cohort study in patients 60 years or older with CD, there were no differences in adverse events between patients on risankizumab and vedolizumab. Compared to risankizumab, patients on ustekinumab or anti-TNFs had a higher likelihood of infection. This suggest that risankizumab or vedolizumab may be safer options for older adults with CD.
Forest plots generated by ChatGPT.

Figure: Figure 1: Forest plots of adverse events between 31 days and one year of therapy initiation in patients on risankizumab as compared to anti-TNFs (A), ustekinumab (B), and vedolizumab (C). Major adverse cardiac events (MACE) include myocardial infarction, transient ischemic attacks, and cerebral infarction. DVT: deep vein thrombosis; PE: pulmonary embolism; PNA: pneumonia; URI: upper respiratory tract infection; UTI: urinary tract infection. Forest plots were generated by ChatGPT.
Disclosures:
Anthony Bilotta indicated no relevant financial relationships.
Abbas Rupawala: Abbvie – Consultant, Speakers Bureau. Pfizer – Consultant. Takeda – Consultant.
Anthony Bilotta, MD, PhD1, Abbas Rupawala, MD2. P1161 - Comparing the Safety of Risankizumab vs Other Biological Therapies in Older Adults With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

